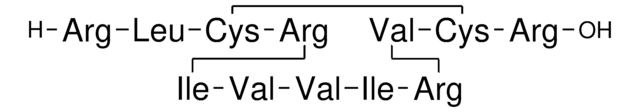C5241
Cinnamycin
from Streptomyces cinnamoneus, ≥95% (HPLC)
Synonym(e):
Lanthiopeptin, NSC-71936, Ro 09-0198
Größe auswählen
About This Item
Empfohlene Produkte
Biologische Quelle
Streptomyces cinnamoneus
Qualitätsniveau
Assay
≥95% (HPLC)
Form
solid
Löslichkeit
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
Wirkungsspektrum von Antibiotika
fungi
Wirkungsweise
cell membrane | interferes
Lagertemp.
2-8°C
SMILES String
S1[C@@H](C2NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H]3NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]5NC(=O)[C@@H](NC(=O)[C@H]7N(CCC7)C(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C1)N)CCCN\C(=N/[H])\N)CCC(=O)N)CS
InChI
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
InChIKey
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Verwandte Kategorien
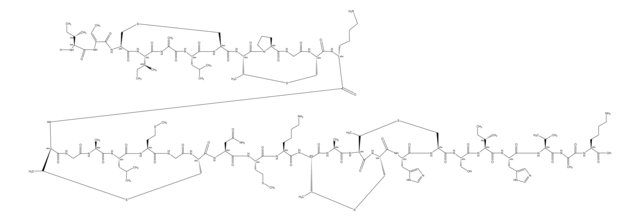
Amino Acid Sequence
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
Active Filters
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.