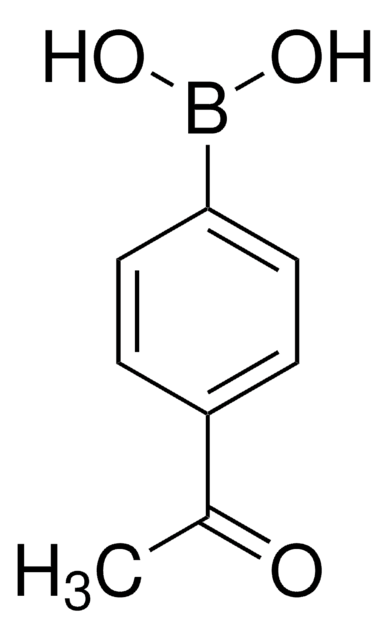
594539
4-Methoxycarbonylphenylborsäure
≥95%
Synonym(e):
Methyl-4-boron-benzoat
About This Item
Empfohlene Produkte
Assay
≥95%
Form
powder
mp (Schmelzpunkt)
197-200 °C (lit.)
SMILES String
COC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO4/c1-13-8(10)6-2-4-7(5-3-6)9(11)12/h2-5,11-12H,1H3
InChIKey
PQCXFUXRTRESBD-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- One-pot ipso-nitration of arylboronic acids
- Copper-catalyzed nitration
- Cyclocondensation followed by palladium-phosphine-catalyzed Suzuki-Miyaura coupling
- Reagent used in Preparation of
- Biaryls via nickel-catalyzed Suzuki-Miyaura cross-coupling reaction of aryl halides with arylboronic acid†
- Chromenones and their bradykinin B1 antagonistic activit†
- Pt nanoparticles@Photoactive metal-organic frameworks resulting in efficient hydrogen evolution via synergistic photoexcitation and electron injectio†
- Salicylate-based thienylbenzoic acids as E. coli methionine aminopeptidase inhibitor†
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.





![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)