Alle Fotos(1)
Wichtige Dokumente
295337
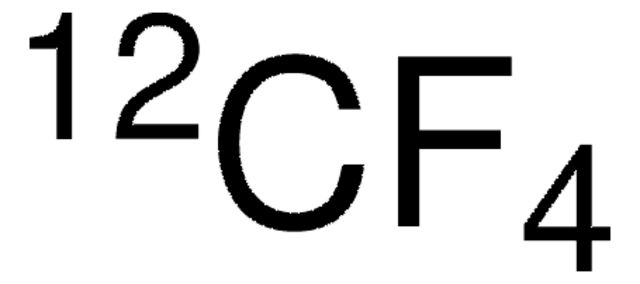
Trifluormethan
≥98%
Synonym(e):
Fluorform, HFC-23
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CHF3
CAS-Nummer:
Molekulargewicht:
70.01
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12142100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Dampfdichte
2.43 (vs air)
Dampfdruck
635 psi ( 21 °C)
Assay
≥98%
bp
−84 °C (lit.)
mp (Schmelzpunkt)
−160 °C (lit.)
SMILES String
FC(F)F
InChI
1S/CHF3/c2-1(3)4/h1H
InChIKey
XPDWGBQVDMORPB-UHFFFAOYSA-N
Verpackung
Packaged in carbon steel lecture bottles with CGA 110F/180M Al-Si-Bronze needle valve.
Sonstige Hinweise
See Technical Information Bulletin AL-151 Gas Regulators: Selection, Installation, and Operation
Rechtliche Hinweise
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
Empfehlung
Produkt-Nr.
Beschreibung
Preisangaben
Empfohlenes Ventil
Entlüftungsventil
Produkt-Nr.
Beschreibung
Preisangaben
Regulierungsbehörde
Schlaucholive
Produkt-Nr.
Beschreibung
Preisangaben
auch häufig zusammen mit diesem Produkt gekauft
Produkt-Nr.
Beschreibung
Preisangaben
Signalwort
Warning
H-Sätze
P-Sätze
Gefahreneinstufungen
Press. Gas Liquefied gas
Lagerklassenschlüssel
2A - Gases
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Mark A Honey et al.
The Journal of organic chemistry, 77(3), 1396-1405 (2012-01-24)
Ethyl 2-diazo-4,4,4-trifluoro-3-oxobutanoate is a highly versatile intermediate for the synthesis of a wide range of trifluoromethyl heterocycles. With the use of rhodium(II) or copper(II) catalyzed carbene X-H insertion reactions as key steps, a diverse set of trifluoromethyl-oxazoles, -thiazoles, -imidazoles, -1,2,4-triazines
Shun Noritake et al.
Organic & biomolecular chemistry, 7(17), 3599-3604 (2009-08-14)
Chiral nonracemic guanidines act as Brønsted bases to generate guanidinium enolates for the enantioselective electrophilic trifluoromethylation of beta-keto esters by means of S-(trifluoromethyl)dibenzothiophenium tetrafluoroborate (Umemoto reagent) with good enantioselectivity of 60-70% range. Despite the fact that the ees are still
Thierry Milcent et al.
Organic & biomolecular chemistry, 8(13), 3025-3030 (2010-05-12)
Trifluoromethyl nitrones were obtained in high yields by condensation of various hydroxylamines with trifluoroacetaldehyde hydrate. The nucleophilic diastereoselective additions of organometallic reagents to these nitrones afforded the corresponding optically active trifluoroethyl hydroxylamines in good yields.
Yongwei Wu et al.
Journal of the American Chemical Society, 134(35), 14334-14337 (2012-08-22)
A new approach toward the asymmetric synthesis of optically active trifluoromethylated amines was enabled by an unprecedented, highly enantioselective catalytic isomerization of trifluoromethyl imines with a new chiral organic catalyst. Not only aryl but also alkyl trifluoromethylated amines could be
Charles S Thomoson et al.
The Journal of organic chemistry, 78(17), 8904-8908 (2013-08-13)
Fluoroform, CHF3, a non-ozone-depleting, nontoxic, and inexpensive gas can be used as a difluorocarbene source in a process for the conversion of phenols and thiophenols to their difluoromethoxy and difluorothiomethoxy derivatives. The reactions are carried out at moderate temperatures and
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.