推薦產品
等級
pharmaceutical primary standard
API 家族
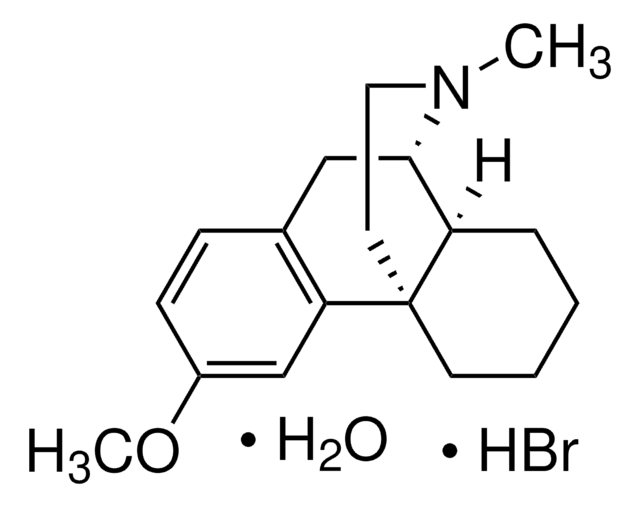
dextromethorphan
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
N1([C@@H]2[C@@H]3[C@@](CC1)(CCCC3)c4c(ccc(c4)OC)C2)C
InChI
1S/C18H25NO/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18/h6-7,12,15,17H,3-5,8-11H2,1-2H3/t15-,17+,18+/m1/s1
InChI 密鑰
MKXZASYAUGDDCJ-NJAFHUGGSA-N
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Dextromethorphan USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Aquatic Chronic 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Eric Guenin et al.
Clinical drug investigation, 34(9), 609-616 (2014-07-17)
Dextromethorphan hydrobromide (DM) is a widely used antitussive. This study determined, for the first time, the basic pharmacokinetic profile of DM and its active metabolite, dextrorphan (DP) in children and adolescents. Thirty-eight male and female subjects at risk for developing
Rüdiger Kaspera et al.
Biochemical pharmacology, 91(1), 109-118 (2014-06-29)
Ritonavir, an HIV protease inhibitor, is successfully used for the prevention and treatment of HIV infections. Ritonavir pharmacokinetics are complicated by inhibition, induction and pharmacogenetics of cytochrome P450 (CYP) enzymes mediating its clearance. This investigation revealed that CYP2J2, along with
Jasbir D Upadhyaya et al.
PloS one, 9(10), e110373-e110373 (2014-10-24)
Activation of bitter taste receptors (T2Rs) in human airway smooth muscle cells leads to muscle relaxation and bronchodilation. This finding led to our hypothesis that T2Rs are expressed in human pulmonary artery smooth muscle cells and might be involved in
Sang Yoon Lee et al.
Toxicology letters, 229(1), 33-40 (2014-06-10)
Although cytochrome P450 inhibition is the major drug-drug interaction (DDI) mechanism in clinical pharmacotherapy, DDI of a number of well-established drugs have not been investigated. Rifampicin, isoniazid, pyrazinamide and ethambutol combination therapy inhibits clearance of theophylline in patients with tuberculosis.
Sang Yoon Lee et al.
Xenobiotica; the fate of foreign compounds in biological systems, 45(2), 131-138 (2014-08-26)
1. The herb-drug interaction potential of Hwang-Ryun-Hae-Dok-Tang (HR) extracts mediated by cytochrome P450 (CYP) inhibition was determined using human liver microsomes. 2. HR strongly inhibited CYP1A2 and moderately inhibited CYP2C19, CYP2D6, and CYP3A4 (testosterone) but not CYP2A6, CYP2B6, CYP2C8, CYP2C9, and CYP3A4
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務