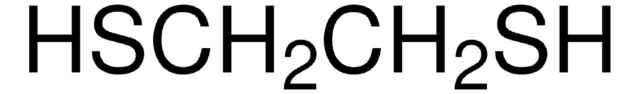推薦產品
品質等級
產品線
ReagentPlus®
化驗
≥99%
折射率
n20/D 1.587 (lit.)
bp
188 °C (lit.)
mp
−15 °C (lit.)
密度
1.057 g/mL at 20 °C (lit.)
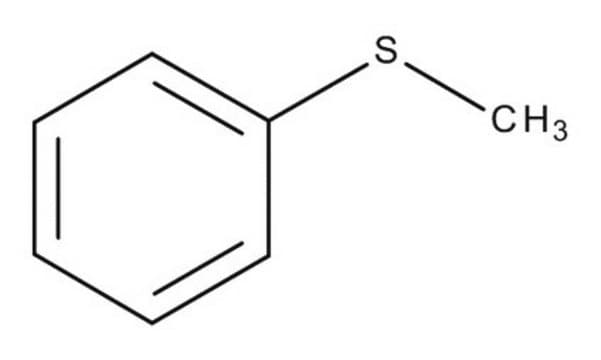
官能基
thioether
SMILES 字串
CSc1ccccc1
InChI
1S/C7H8S/c1-8-7-5-3-2-4-6-7/h2-6H,1H3
InChI 密鑰
HNKJADCVZUBCPG-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
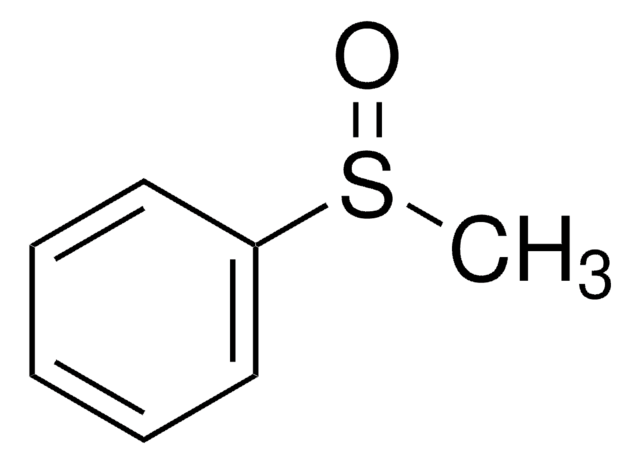
硫代苯甲醚可用于通过氧化合成甲基苯基亚砜。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險分類
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
163.4 °F - closed cup
閃點(°C)
73 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Mild and selective oxidation of sulfur compounds in trifluoroethanol: diphenyl disulfide and methyl phenyl sulfoxide.
Ravikumar KS, et al.
Organic Syntheses, 184-189 (2003)
Methyl phenyl sulfoxide.
Johnson CR & Keiser JE.
Organic Syntheses, 78-78 (1966)
Jiyun Park et al.
Journal of the American Chemical Society, 133(14), 5236-5239 (2011-03-18)
The mechanism of sulfoxidation of thioaniosoles by a non-heme iron(IV)-oxo complex is switched from direct oxygen transfer to metal ion-coupled electron transfer by the presence of Sc(3+). The switch in the sulfoxidation mechanism is dependent on the one-electron oxidation potentials
Rémy Ricoux et al.
Organic & biomolecular chemistry, 7(16), 3208-3211 (2009-07-31)
Two new artificial hemoproteins or "hemozymes", obtained by non covalent insertion of Fe(III)-meso-tetra-p-carboxy- and -p-sulfonato-phenylporphyrin into xylanase A from Streptomyces lividans, were characterized by UV-visible spectroscopy and molecular modeling studies, and were found to catalyze the chemo- and stereoselective oxidation
Jun-Long Zhang et al.
Chemical communications (Cambridge, England), (14)(14), 1665-1667 (2008-03-28)
We demonstrate that incorporation of MnSalen into a protein scaffold enhances the chemoselectivity in sulfoxidation of thioanisole and find that both the polarity and hydrogen bonding of the protein scaffold play an important role in tuning the chemoselectivity.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務