全部照片(1)
About This Item
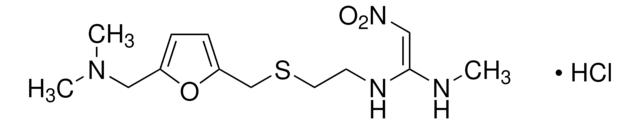
經驗公式(希爾表示法):
C13H22N4O3S · HCl
CAS號碼:
分子量::
350.86
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
暫時無法取得訂價和供貨情況
推薦產品
等級
pharmaceutical primary standard
API 家族
ranitidine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
Cl[H].CN\C(NCCSCc1ccc(CN(C)C)o1)=C\[N+]([O-])=O
InChI
1S/C13H22N4O3S.ClH/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3;/h4-5,9,14-15H,6-8,10H2,1-3H3;1H/b13-9-;
InChI 密鑰
GGWBHVILAJZWKJ-CHHCPSLASA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ranitidine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
H2 组胺受体拮抗剂;抗溃疡剂。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
T S Gaginella et al.
Drug intelligence & clinical pharmacy, 17(12), 873-885 (1983-12-01)
Ranitidine is a selective, competitive histamine H2-receptor antagonist recently approved by the Food and Drug Administration for use in the short-term treatment of active duodenal ulcers and gastric hypersecretory conditions. Ranitidine is four to ten times more potent than cimetidine
Question 1: does the use of ranitidine increase the risk of NEC in preterm infants?
Manigandan Chandrasekaran et al.
Archives of disease in childhood, 99(4), 390-392 (2014-03-15)
M B Evans et al.
Journal of pharmaceutical and biomedical analysis, 7(1), 1-22 (1989-01-01)
The selection, development, definition and validation of selective stability-indicating procedures for high-performance liquid chromatographic and thin-layer chromatographic analyses of ranitidine hydrochloride are described. The procedures used in conjunction can be applied to the quality assurance and stability assessments of both
[The development of hemorrhagic vasculitis (Schönlein-Henoch disease) in a patient with a chronic stomach ulcer against a background of Sostril (ranitidine hydrochloride) therapy].
E N Glazunov et al.
Vestnik khirurgii imeni I. I. Grekova, 152(1-2), 102-103 (1994-01-01)
Natalia Alonso et al.
The Biochemical journal, 459(1), 117-126 (2014-01-15)
7TMRs (seven-transmembrane receptors) exist as conformational collections in which different conformations would lead to differential downstream behaviours such as receptor phosphorylation, G-protein activation and receptor internalization. In this context, a ligand may cause differential activation of some, but not all
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務