推薦產品
化驗
>99% (TLC)
形狀
liquid
包裝
pkg of 1 × 1 mL (810603C-1mg)
製造商/商標名
Avanti Research™ - A Croda Brand 810603C
濃度
1 mg/mL (810603C-1mg)
脂質類型
ESR probes
phospholipids
運輸包裝
dry ice
儲存溫度
−20°C
一般說明
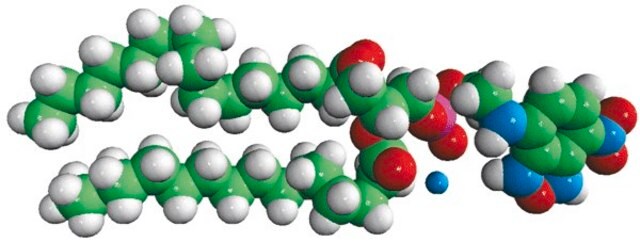
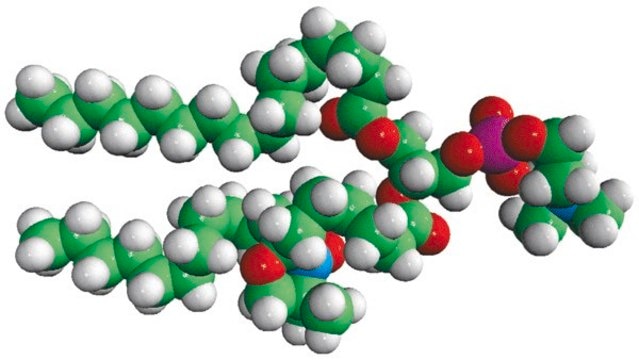
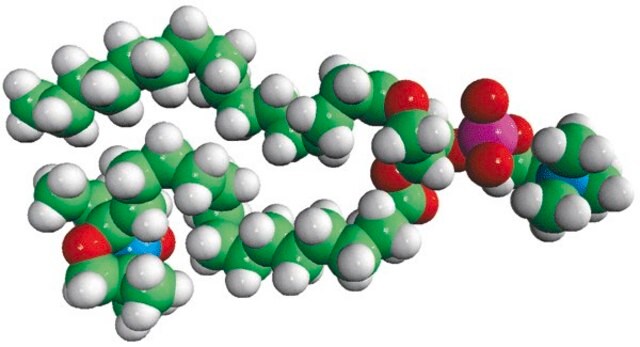
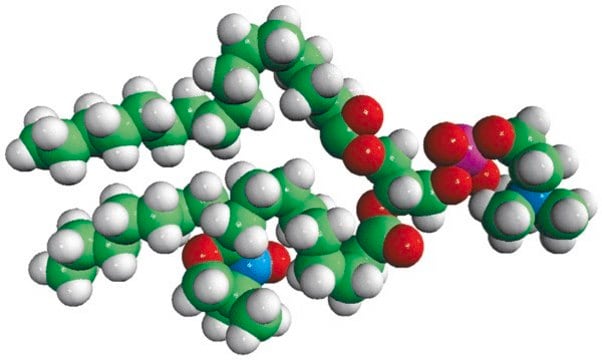
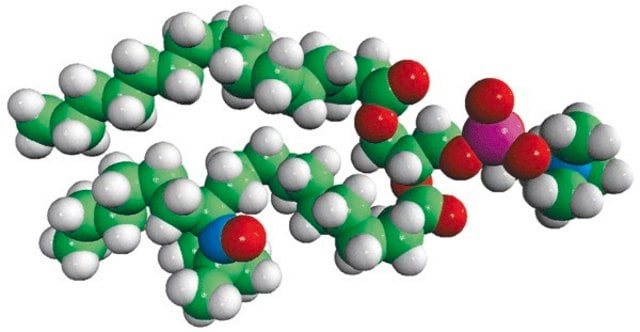
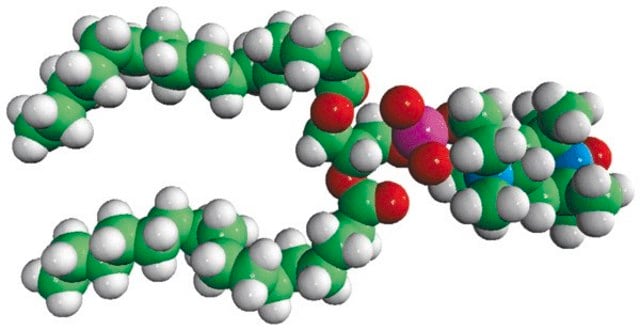
Avanti′s nitroxide spin product listing is a group of compounds designed to act as membrane probes. A variety of positions down the hydrophobic chain are labeled with the nitroxide functional groups to allow probing the membrane at various depths. These compounds have been synthesized from 1-palmitoyl-2-hydroxy-sn-glycerol-3-phosphocholine with the product being purified by column chromatography. Various n-doxyl phosphocholines have been recently used as biophysical tools to elucidate membrane trafficking with phosphatidylinositol transfer proteins and as fluorescent quenchers in lipid bilayer structural studies.[1][2]
Phosphocholine is considered as a precursor molecule. It is formed during the breakdown of phosphatidylcholine metabolism.[3]
應用
生化/生理作用
Phosphatidylcholine (PC) lowers the levels of cholesterol and triglycerides.[6]
包裝
5 mL Clear Glass Sealed Ampule (810603C-1mg)
準備報告
Product use: To prevent aggregation, prepare water-based solutions of 2 mM stock solutions of n-DOXYL PCs and store in plastic. Dilute stock solutions to 0.03- 0.1 mM solutions for EPR studies.[7] For liposome preparations in fluorescent quenching measurements, dissolve the doxyl lipid in 150 μl absolute ethanol for a concentration of 40.3 mM [2], Additional supplemental information.
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
標靶器官
Central nervous system, Liver,Kidney
水污染物質分類(WGK)
WGK 3
Biology-cancer metabolic phenotype
NMR Metabolomics in Cancer Research, 15-138 (2013)
Fluorescence studies on the interaction of a synthetic signal peptide and its analog with liposomes.
Q Wang et al.
Biochimica et biophysica acta, 1324(1), 69-75 (1997-02-21)
The N-terminal signal sequence of glucitol permease of Escherichia coli (Gut22: MIETITPGAVWFIGLFQKGGEC) and its analog (Gut22Ana: MIETITHGAEWFIGLFQKGGEC) were synthesized. The analog had a Pro residue substituted for the His at the 7th position of Gut22 and a Val residue substituted
L A Falls et al.
The Journal of biological chemistry, 276(26), 23895-23902 (2001-04-20)
The hydrophobic omega-loop within the prothrombin gamma-carboxyglutamic acid-rich (Gla) domain is important in membrane binding. The role of this region in membrane binding was investigated using a synthetic peptide, PT-(1-46)F4W, which includes the N-terminal 46 residues of human prothrombin with
Phosphatidylserine dynamics in cellular membranes.
Kay, J.G
Molecular Biology of the Cell, 23, 2198-2212 (2012)
Insight into antibody combining sites using nuclear magnetic resonance and spin label haptens
McConnell HM
Advances in Protein Chemistry, 49, 135-148 (1996)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務