推薦產品
化驗
95%
形狀
(Liquid or Semi-Solid or Paste or Solid)
反應適用性
reaction type: click chemistry
reagent type: cross-linking reagent
官能基
azide
carboxylic acid
儲存溫度
2-8°C
SMILES 字串
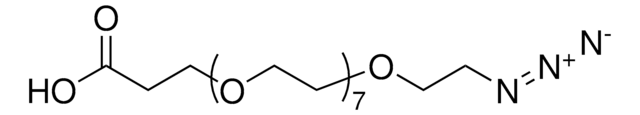
OC(COCCOCCOCCOCCOCCOCCN=[N+]=[N-])=O
應用
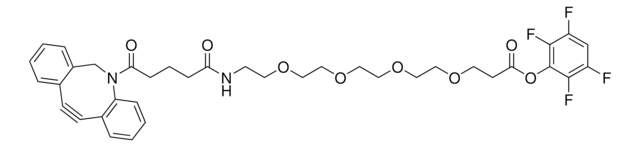
This heterobifunctional, PEGylated crosslinker 20-Azido-3,6,9,12,15,18-hexaoxaicosanoic acid features a carboxyl group at one end and azide at the other. The hydrophillic PEG linker facilitates solubility in biological applications. This azido-PEG6-acid linker can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation.
法律資訊
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
相關產品
產品號碼
描述
訂價
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Yong Ma et al.
Bioconjugate chemistry, 21(11), 1994-1999 (2010-10-14)
A surface-bound cytomimetic assembly based on chemically selective and biocompatible immobilization and further modification of intact liposome is described. Liposomes carrying PEG-triphenylphosphine were chemoselectively immobilized onto azide-modified glass slides through Staudinger ligation, followed by modification with azide-modified lactose as a
Ryota Sato et al.
Journal of the American Chemical Society, 139(48), 17397-17404 (2017-11-10)
Single-molecule imaging (SMI) has been widely utilized to investigate biomolecular dynamics and protein-protein interactions in living cells. However, multicolor SMI of intracellular proteins is challenging because of high background signals and other limitations of current fluorescence labeling approaches. To achieve
Towards potential nanoparticle contrast agents: Synthesis of new functionalized PEG bisphosphonates.
Souad Kachbi-Khelfallah et al.
Beilstein journal of organic chemistry, 12, 1366-1371 (2016-08-26)
The use of nanotechnologies for biomedical applications took a real development during these last years. To allow an effective targeting for biomedical imaging applications, the adsorption of plasmatic proteins on the surface of nanoparticles must be prevented to reduce the
Joel Hwang et al.
Chemical communications (Cambridge, England), 50(24), 3159-3162 (2014-01-30)
Oligo(ethylene glycol)-linked light fluorous tags have been found to be optimal for conjugating to glycans for both high-yield enzymatic glycosylation reactions using one-pot multienzyme (OPME) systems and quick product purification using fluorous solid-phase extraction (FSPE) cartridges. The combination of OPME
Mingcheng Qian et al.
ChemMedChem, 13(9), 944-956 (2018-02-17)
Currently, there is mounting evidence that intermolecular receptor-receptor interactions may result in altered receptor recognition, pharmacology and signaling. Heterobivalent ligands have been proven useful as molecular probes for confirming and targeting heteromeric receptors. This report describes the design and synthesis
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務