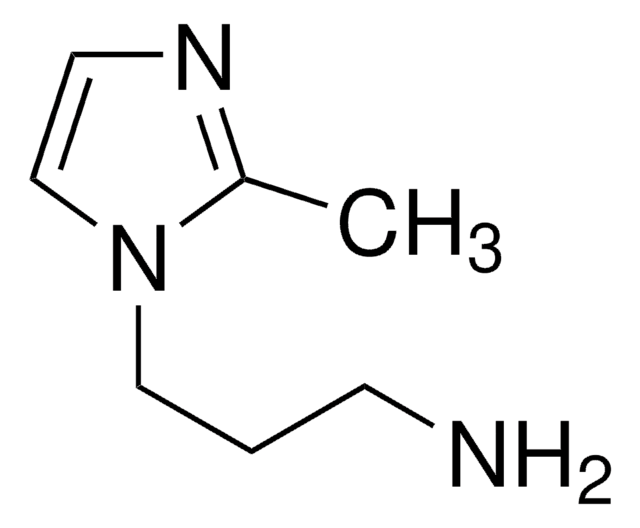
推薦產品
品質等級
化驗
≥97%
形狀
liquid
折射率
n20/D 1.519 (lit.)
密度
1.049 g/mL at 25 °C (lit.)
官能基
amine
SMILES 字串
NCCCn1ccnc1
InChI
1S/C6H11N3/c7-2-1-4-9-5-3-8-6-9/h3,5-6H,1-2,4,7H2
InChI 密鑰
KDHWOCLBMVSZPG-UHFFFAOYSA-N
應用
1-(3-氨基丙基)咪唑被用于合成pH敏感性聚天冬酰胺衍生物。它还被用于通过接枝反应制备具有不同程度octaceylamine (C18)取代的pH敏感性两亲聚合物。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
309.2 °F
閃點(°C)
154 °C
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Abootaleb Sedighi et al.
Talanta, 186, 568-575 (2018-05-23)
Several solution-based methods have recently been adapted for use in paper substrates for enzymatic amplification to increase the number of copies of DNA sequences. There is limited information available about the impact of a paper matrix on DNA amplification by
Kwangwon Seo et al.
Journal of nanoscience and nanotechnology, 10(10), 6986-6991 (2010-12-09)
The effect of hydrophobic octadecyl groups on pH-dependent aggregation behavior of polyaspartammide derivatives was studied. A series of pH-sensitive amphiphilic polymers with different degrees of octaceylamine (C18) substitution were synthesized through a successive graft reaction of octaceylamine, O-(2-aminoethyl)-O'-methylpolyethylene glycol, and
Taehoon Sim et al.
Pharmaceutics, 12(9) (2020-09-06)
Combination therapy is considered to be a promising strategy for improving the therapeutic efficiency of cancer treatment. In this study, an on-demand pH-sensitive nanocluster (NC) system was prepared by the encapsulation of gold nanorods (AuNR) and doxorubicin (DOX) by a
Di Lu et al.
AAPS PharmSciTech (2018-06-20)
The complex design of multifunctional nanomedicine is beneficial to overcome the multiple biological barriers of drug delivery, but it also presents additional hurdles to clinical translation (e.g., scaling-up and quality control). To address this dilemma, we employed a simple imidazole-bearing
Kwangwon Seo et al.
Macromolecular bioscience, 6(9), 758-766 (2006-09-13)
New pH-sensitive polyaspartamide derivatives were synthesized by grafting 1-(3-aminopropyl)imidazole and/or O-(2-aminoethyl)-O'-methylpoly(ethylene glycol) 5000 on polysuccinimide for application in intracellular drug delivery systems. The DS of 1-(3-aminopropyl)imidazole was adjusted by the feed molar ratio, and the structure of the prepared polymer
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務