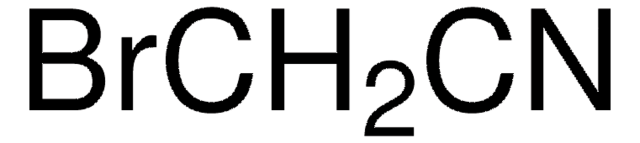推薦產品
形狀
liquid
濃度
1.5 M in THF
密度
0.893 g/mL at 25 °C
SMILES 字串
[Li]N([Si](C)(C)C)[Si](C)(C)C
InChI
1S/C6H18NSi2.Li/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1
InChI 密鑰
YNESATAKKCNGOF-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Lithium bis(trimethylsilyl)amide is commonly used in organic synthesis as a non-nucleophilic strong Bronsted base. It is soluble in most nonpolar solvents such as aromatic hydrocarbons, hexanes, and THF.
應用
Lithium bis(trimethylsilyl)amide can be used as a reagent:
- In the deprotonation and nucleophilic difluoromethylation reactions.
- 3-methoxy substituted dihydropyrrole derivatives by reacting with aldehydes and lithiated methoxyallene via in situ formations of N-trimethylsilylated imines.
- In Darzens condensation and directed aldol condensation reactions.
- To synthesize poly(N-octyl-p-benzamide)s by chain-growth polycondensation of 4-octylaminobenzoic acid methyl ester.
訊號詞
Danger
危險分類
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Self-heat. 1 - Skin Corr. 1B - STOT SE 3
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
31.3 °F - closed cup
閃點(°C)
-0.4 °C - closed cup
客戶也查看了
Daniele Fabbri et al.
Environmental science & technology, 42(8), 2957-2963 (2008-05-24)
Using the pyrolysis-gas chromatography-mass spectrometry and off-line pyrolysis/silylation methods for lignites from three Miocene brown coal basins of Poland resulted in the characterization of many organic compounds, including dominant cellulose degradation products such as levoglucosan, 1,6-anhydro-beta-D-glucofuranose, and 1,4:3,6-dianhydroglucopyranose. Levoglucosan is
Hexamethyldisilazane-mediated controlled polymerization of alpha-amino acid N-carboxyanhydrides.
Hua Lu et al.
Journal of the American Chemical Society, 129(46), 14114-14115 (2007-10-30)
Jerry Isaacson et al.
Angewandte Chemie (International ed. in English), 48(10), 1845-1848 (2009-01-29)
(-)-Dysibetaine has been synthesized in 11 steps from readily available L-malic acid (see scheme). The key step is a unique Ugi 4-center-3-component cyclization reaction, where an ester group acts as the carboxylic acid component. The use of 1,1,1,3,3,3-hexamethyldisilazane as an
A Sebok et al.
Journal of chromatography. A, 1211(1-2), 104-112 (2008-10-14)
This paper presents a derivatization, mass fragmentation study relating to the most common six cholic acids, such as cholic, lithocholic, chenodeoxycholic, ursodeoxycholic, 3-hydroxy,7-ketocholanic and dehydrocholic acids, identified and quantified as pollutants in the aquatic environment at the first time. Derivatizations
Natalie M Clark et al.
Chemical communications (Cambridge, England), (39)(39), 5835-5837 (2009-09-30)
'Conventional' (-)-sparteine adducts of lithium and sodium 1,1,1,3,3,3-hexamethyldisilazide (HMDS) were prepared and characterised, along with an unexpected and 'unconventional' hydroxyl-incorporated sodium sodiate, [(-)-sparteine x Na(mu-HMDS)Na x (-)-sparteine](+)[Na(4)(mu-HMDS)(4)(OH)](-)--the complex anion of which is the first inverse crown ether anion.
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務