推薦產品
化驗
98%
光學活性
[α]20/D +4°, c = 1.0242 in chloroform stab. with amylenes
mp
103-107 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m1/s1
InChI 密鑰
CESUXLKAADQNTB-SSDOTTSWSA-N
一般說明
應用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
文章
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Ellman 的亞磺酰胺有對映體和外消旋體兩種形式,可供您進行研究。這種多用途的有用輔助劑在學術界和工業界都有廣泛的應用。
相關內容
The Ellman group has participated in the development of a variety of C-H functionalization methods. An electron rich phosphine ligand has proven to be very useful for a variety of Rh(I)-catalyzed C-C bond forming reactions applicable to heterocycle synthesis as exemplified in the recent Science paper “Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis.” Another useful ligand developed for the highly functional group compatible direct arylation of nitrogen heterocycles is described in a 2008 J. Am. Chem. Soc. paper “Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope through Mechanistic Insight.” The Ellman group also developed the chiral amine reagent tert-Butanesulfinamide, which is extensively used in academics and industry for the asymmetric synthesis of amines. A comprehensive survey of tert-Butanesulfinamide methods and applications up through 2009 is provided in the 2010 Chemical Reviews article, “Synthesis and Applications of tert-Butanesulfinamide.”
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務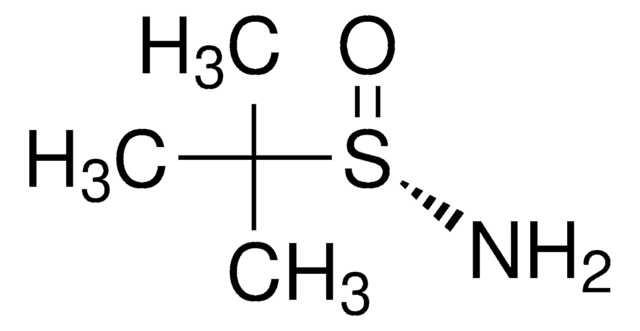








![(R)-N-[(1R,2R)-2-(3-(3,5-双(三氟甲基)苯基)脲基)环己基]-叔丁基亚磺酰胺 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)