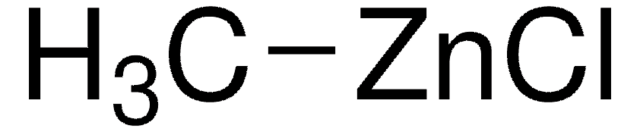推薦產品
形狀
liquid
濃度
1.0 M in heptane
bp
44-46 °C
密度
0.724 g/mL at 25 °C
SMILES 字串
C[Zn]C
InChI
1S/2CH3.Zn/h2*1H3;
InChI 密鑰
AXAZMDOAUQTMOW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Dimethylzinc (Zn(CH3)2) is a methylating reagent used to prepare methylated organic compounds as well as organometallic compounds containing methyl groups . It is also utilized as a reagent to prepare amino alcohols, oximes, and hydrazones from arylamines, alkoxyamines, and dialkylhydrazines respectively, by radical addition reaction in the presence of air and THF.
Dimethylzinc is a diorganozinc reagent and nucleophile used in the synthesis of propargylic amines.
Dimethylzinc is a diorganozinc reagent and nucleophile used in the synthesis of propargylic amines.
應用
Dimethylzinc solution can be used as:
- A catalyst with nickel for the stereoselective C−2 alkenylation and dialkenylation of pyridine derivatives by alkynes to give monoalkenylation products.
- A reagent with aldehydes and 2-methoxyaniline for the synthesis of enantioselective alkyl and aralkyl secondary amines via one-pot three-component coupling reaction in the presence of zirconium tetraisopropoxide.
- A methylating reagent for methylation of fluoroalkylated pyruvates in the presence of copper/chiral diphosphine catalyst.
訊號詞
Danger
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 2
標靶器官
Central nervous system
儲存類別代碼
4.2 - Pyrophoric and self-heating hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
30.2 °F - closed cup
閃點(°C)
-1 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Kohsuke Aikawa et al.
Beilstein journal of organic chemistry, 14, 576-582 (2018-04-07)
The catalytic asymmetric methylation of fluoroalkylated pyruvates is shown with dimethylzinc as a methylating reagent in the presence of a copper catalyst bearing a chiral phosphine ligand. This is the first catalytic asymmetric methylation to synthesize various α-fluoroalkylated tertiary alcohols
Formation of transient dimethylzinc, dimethylcadmium, and dimethyllead species via methylation of zinc (2+), cadmium (2+), and lead (2+) by a trans-dimethylcobalt complex
Witman MW and Weber JH
Inorganic Chemistry, 16(10), 2512-2515 (1977)
Tito Akindele et al.
Accounts of chemical research, 42(2), 345-355 (2008-12-31)
Developments in modern organic synthesis owe much to the field of radical chemistry. Mild reaction conditions, high selectivity, good functional group tolerance and high product yield are features that have made reactions involving radical species indispensable tools for synthetic chemists.
Dimethylzinc-mediated alkynylation of imines
Lorenzo Z et al.
The Journal of Organic Chemistry, 71, 1558-1562 (2006)
Diethylzinc
Georges-Pierre and E
Synlett, 1937-1938 (2014)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務