推薦產品
化驗
99%
mp
178-180 °C (lit.)
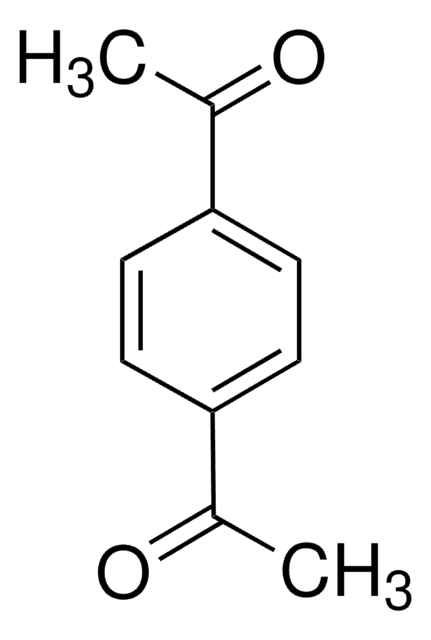
官能基
ketone
SMILES 字串
CC(=O)c1cc(C(C)=O)c(O)cc1O
InChI
1S/C10H10O4/c1-5(11)7-3-8(6(2)12)10(14)4-9(7)13/h3-4,13-14H,1-2H3
InChI 密鑰
GEYCQLIOGQPPFM-UHFFFAOYSA-N
一般說明
應用
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) may be used in the synthesis of the following:
- Schiff base ligands[4][1][5][6]
- hexadentate chalcogenated bisimine ligands[7]
- 1,5-benzodiazepines[8]
- ketimine of chitosan[9]
- mannich bases[10]
- hydrazone ligands[11]
- thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands[12]
- binuclear cobalt(II) and copper(II) complexes[13]
- europium (III) complexes[14]
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
M Shebl et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(1), 428-436 (2009-12-08)
Mono- and binuclear VO(IV), Ce(III), Th(IV) and UO(2)(VI) complexes of thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands derived from 4,6-diacetylresorcinol were synthesized. The structures of these complexes were elucidated by elemental analyses, IR, UV-vis, ESR, (1)H NMR and mass spectra as well
Structure of 4, 6-diacetylresorcinol.
Kokila MK, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(6), 1133-1134 (1992)
Cahit Demetgül
Carbohydrate polymers, 89(2), 354-361 (2012-06-20)
In this study, a new chitosan derivative (ketimine) was synthesized by condensation of chitosan with 4,6-diacetylresorcinol (DAR) at heterogeneous medium. The ketimine derivative of chitosan (DAR-chitosan) was characterized by elemental (C, H, N), spectral (DR-UV-vis and FT-IR spectroscopy), structural (powder
Arun Kumar et al.
Journal of hazardous materials, 269, 9-17 (2013-12-10)
Potentially hexadentante [O(-),N,E:E,N,O(-)] chalcogenated bisimine ligands L1-L3 have been synthesized by reaction of 1,1'-(4,6-dihydroxy-1,3-phenylene)bisethanone with H2N(CH2)2SPh, H2N(CH2)2SePh and H2N(CH2)2TeC6H4-4-OMe respectively. The L1-L3 react with Na2PdCl4 resulting in their partial hydrolysis, which appears to be metal-promoted. Of the two [(CH3)CN(CH2)2EAr] fragments
Magdy Shebl et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 113, 356-366 (2013-06-08)
Reactions of 4,6-diacetylresorcinol with different cobalt(II) and copper(II) salts viz., OAc(-), Cl(-), NO3(-) and SO4(2-), yielded a new series of binuclear metal complexes. Reactions of the ligand with these metal ions in the presence of a secondary ligand (L') [O,O-donor;
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務