全部照片(4)
About This Item
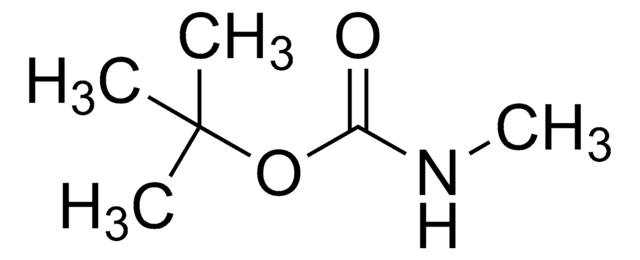
線性公式:
CH3C6H4SO2NHCO2C(CH3)3
CAS號碼:
分子量::
271.33
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
mp
121-123 °C (lit.)
溶解度
chloroform: soluble 25 mg/mL, clear, colorless
SMILES 字串
Cc1ccc(cc1)S(=O)(=O)NC(=O)OC(C)(C)C
InChI
1S/C12H17NO4S/c1-9-5-7-10(8-6-9)18(15,16)13-11(14)17-12(2,3)4/h5-8H,1-4H3,(H,13,14)
InChI 密鑰
DUTLOVSBVBGNDM-UHFFFAOYSA-N
一般說明
在 Mitsunobu 条件下可直接与伯醇、仲醇和烯丙醇发生偶联反应生成各种磺酰基保护胺。
應用
N-(tert-Butoxycarbonyl)-p-toluenesulfonamide may be used in the preparation of enyne amide, precursor for Pauson-Khand reaction.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Tetrahedron Letters, 30, 5709-5709 (1989)
Toshio Honda et al.
The Journal of organic chemistry, 72(17), 6541-6547 (2007-07-31)
Diastereoselective formal synthesis of a monoterpene alkaloid, (-)-incarvilline, the key intermediate for the synthesis of (-)-incarvillateine, was achieved by using an intramolecular Pauson-Khand reaction of (S)-N-[(E)-2-butenyl]-N-(3-butynyl-2-methoxymethoxy)-p-toluenesulfonamide as a key step.
Use of the Mitsunobu reaction in the synthesis of orthogonally protected a, ?-diaminopropionic acids.
Kelleher F.
Tetrahedron Letters, 48(28), 4879-4882 (2007)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務