About This Item
推薦產品
品質等級
化驗
97%
反應適用性
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Hydroformylations
reagent type: ligand
reaction type: Miyaura Borylation Reaction
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
224-228 °C (lit.)
官能基
phosphine
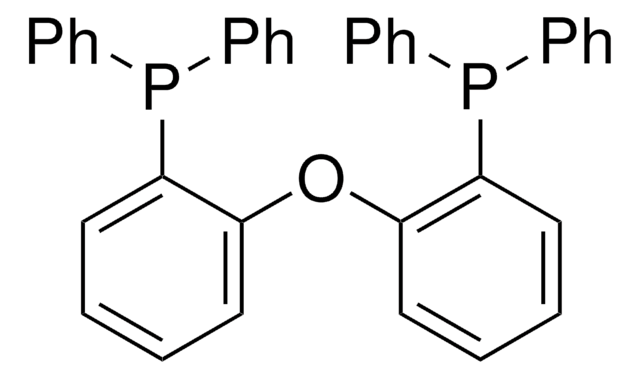
SMILES 字串
CC1(c2c(Oc3c1cccc3P(c4ccccc4)c5ccccc5)c(P(c6ccccc6)c7ccccc7)ccc2)C
InChI
1S/C39H32OP2/c1-39(2)33-25-15-27-35(41(29-17-7-3-8-18-29)30-19-9-4-10-20-30)37(33)40-38-34(39)26-16-28-36(38)42(31-21-11-5-12-22-31)32-23-13-6-14-24-32/h3-28H,1-2H3
InChI 密鑰
CXNIUSPIQKWYAI-UHFFFAOYSA-N
一般說明
对于小规模和高通量应用,可选用ChemBeads形式(928356)
應用
訊號詞
Warning
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務




![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)