推荐产品
表单
powder
颜色
off-white to light yellow
溶解性
0.5 M NaOH: soluble 50 mg/mL
抗生素抗菌谱
Gram-negative bacteria
Gram-positive bacteria
mycoplasma
作用机制
DNA synthesis | interferes
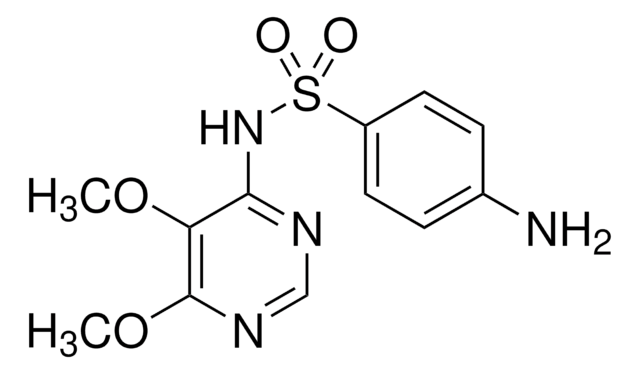
SMILES字符串
Nc1ccc(cc1)S(=O)(=O)Nc2ccc(Cl)nn2
InChI
1S/C10H9ClN4O2S/c11-9-5-6-10(14-13-9)15-18(16,17)8-3-1-7(12)2-4-8/h1-6H,12H2,(H,14,15)
InChI key
XOXHILFPRYWFOD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
其他客户在看
[Sulfachloropyridazine in the treatment of acute infections of the urinary tract in childhood].
R C PEDRINAZZI et al.
Minerva pediatrica, 12, 1342-1347 (1960-10-27)
Absorption and Excretion of Sulfachloropyridazine
Wilfred F. Jones, Jr, Mohsen Ziai, et al.
Exp. Biol. Med, 95, 642-645 (1957)
E van Duijkeren et al.
The Veterinary record, 137(19), 483-486 (1995-11-04)
The pharmacokinetic parameters of a powder formulation of trimethoprim/sulphachlorpyridazine were studied in eight healthy horses which received 5 mg/kg trimethoprim and 25 mg/kg sulphachlorpyridazine 12-hourly with concentrate for five days. The intake of the medicated concentrate by the horses was
Thomas L ter Laak et al.
Environmental toxicology and chemistry, 25(4), 933-941 (2006-04-25)
Environmental exposure assessment of veterinary pharmaceuticals requires estimating the sorption to soil. Soil sorption coefficients of three common, ionizable, antimicrobial agents (oxytetracycline [OTC], tylosin [TYL], and sulfachloropyridazine [SCP]) were studied in relation to the soil properties of 11 different soils.
E van Duijkeren et al.
Journal of veterinary pharmacology and therapeutics, 18(1), 47-53 (1995-02-01)
In the present study, the pharmacokinetic parameters of a trimethoprim/sulphachlorpyridazine preparation following intravenous administration, administration by nasogastric tube and administration with concentrate were determined in the horse. Eight adult horses were dosed at 1 week intervals in a sequentially designed
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门