推荐产品
等級
analytical standard
品質等級
agency
EPA 1694
形狀
powder
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
200-202 °C (lit.)
應用
clinical testing
SMILES 字串
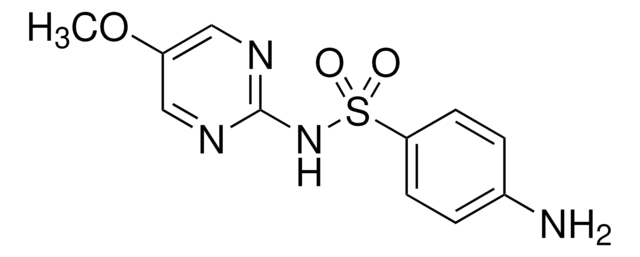
Nc1ccc(cc1)S(=O)(=O)Nc2nccs2
InChI
1S/C9H9N3O2S2/c10-7-1-3-8(4-2-7)16(13,14)12-9-11-5-6-15-9/h1-6H,10H2,(H,11,12)
InChI 密鑰
JNMRHUJNCSQMMB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Sulfathiazole is a broad-spectrum antibacterial drug, belonging to the class of sulfonamides, used in veterinary and human medication for the treatment of infections. It is also involved in promoting growth of livestock and fish.
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Sulfathiazole is used as an analytical reference standard for the quantification of the analyte in meat, and honey samples using different chromatography techniques.
生化/生理作用
通过抑制二氢叶酸合成酶阻断二氢叶酸合成的磺胺类抗生素。
作用方式:在原核生物中抑制叶酸合成。
抗菌谱:革兰氏阳性菌、革兰氏阴性菌、衣原体
抗性机理:二氢叶酸合成酶或叶酸合成替代路径的改变。
作用方式:在原核生物中抑制叶酸合成。
抗菌谱:革兰氏阳性菌、革兰氏阴性菌、衣原体
抗性机理:二氢叶酸合成酶或叶酸合成替代路径的改变。
訊號詞
Warning
危險分類
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
分析证书(COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
其他客户在看
Comparison of separation conditions and ionization methods for the liquid chromatography?mass spectrometric determination of sulfonamides
Kim H-D and Lee WD
Journal of Chromatography A, 984(1), 153-158 (2003)
Determination of sulfonamides in meat by liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry
Kim H-D, et al.
Bull. Korean Chem. Soc., 23(11), 1590-1594 (2002)
Quantitative LC/MS-MS determination of sulfonamides and some other antibiotics in honey
Kaufmann A, et al.
Journal of AOAC (Association of Official Analytical Chemists) International, 85(4), 853-860 (2002)
Merle K Richter et al.
Environmental pollution (Barking, Essex : 1987), 172, 208-215 (2012-10-16)
The effects of sulfathiazole (STA) on Escherichia coli with glucose as a growth substrate was investigated to elucidate the effect-based reaction of sulfonamides in bacteria and to identify biomarkers for bacterial uptake and effect. The predominant metabolite was identified as
Hongyun Niu et al.
Journal of hazardous materials, 190(1-3), 559-565 (2011-04-26)
Humic acid coated Fe(3)O(4) magnetic nanoparticles (Fe(3)O(4)/HA) were prepared for the removal of sulfathiazole from aqueous media. Fe(3)O(4)/HA exhibited high activity to produce hydroxyl (OH) radicals through catalytic decomposition of H(2)O(2). The degradation of sulfathiazole was strongly temperature-dependent and favored
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门