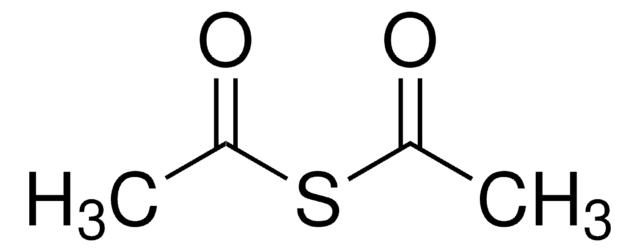
推荐产品
方案
≥98.0%
表单
solid
反应适用性
reaction type: click chemistry
reagent type: ligand
reaction type: Staudinger Reaction
mp
52-55 °C
官能团
phosphine
储存温度
2-8°C
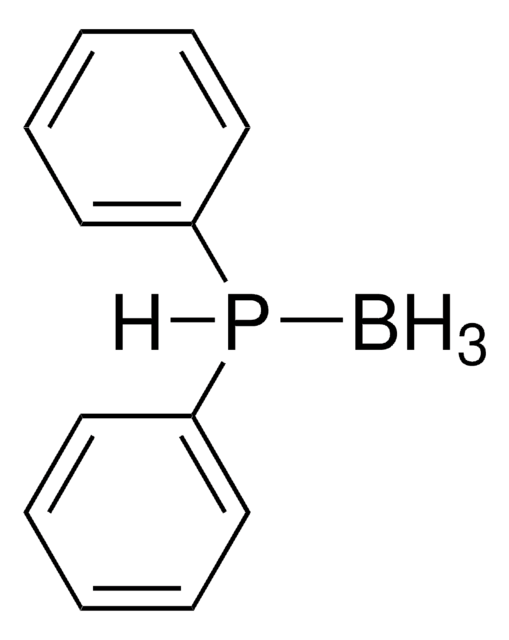
SMILES字符串
B.CC(=O)SCP(c1ccccc1)c2ccccc2
InChI
1S/C15H15OPS.BH3/c1-13(16)18-12-17(14-8-4-2-5-9-14)15-10-6-3-7-11-15;/h2-11H,12H2,1H3;1H3
InChI key
MXPNVFCCEGQGEN-UHFFFAOYSA-N
应用
- Traceless Staudinger ligation reagent with borane protecting group.
- The borane group stabilizes the phosphine against oxidation and can be easily removed with mild basic or acidic conditions to yield the active phosphine.[1]
- After reaction with an azide, the phosphine is eliminated in the presence of water to yield a native amide bond.[2]
- Used in the synthesis of cyclic peptides.[2]

包装
法律信息
相关产品
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
相关内容
Chemoselective ligation strategies are a key success factor for chemical biology research. Ligation techniques open pathways to fully synthetic large peptides and even proteins.
Based on the same working principle as the nontraceless Staudinger Ligation the auxiliary phosphine reagent can be cleaved from the product after the ligation is completed leaving a native amide bond. Thus, the total chemical synthesis of proteins and glycopeptides is enabled overcoming the limitations of native chemical ligation (NCL) of a Cys residue at the ligation juncture.
Raines lab innovations include traceless Staudinger ligation and DTBA reagent, advancing chemical biology research with Sigma-Aldrich.
The reaction between an azide and a phosphine forming an aza-ylide was discovered almost a century ago by Nobel Prize laureate Herrmann Staudinger.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持