595721
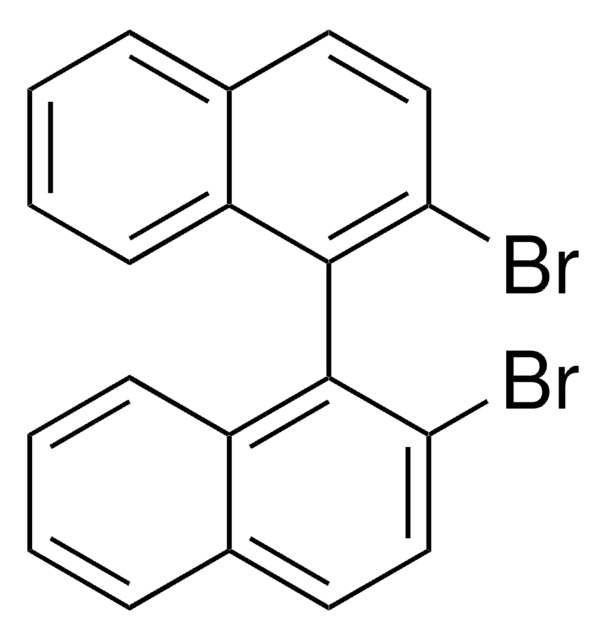
(R)-(+)-3,3′-二溴-1,1′-二-2-萘醇
97%
别名:
(R)-3,3′-Dibromo-1,1′-bi-2-naphthol, (R)-3,3′-Dibromo-[1,1′-Binaphthalene]-2,2′-diol, (R)-Dibromo-1,1′-Bi-2,2′-naphthol, (R)-Dibromo-1,1′-Binaphthalene-2,2′-diol, (R)-Dibromo-1,1-Binaphthol, (R)-Dibromo-BINOL, (R)-Dibromo-bi-2-naphthol
登录查看公司和协议定价
所有图片(2)
About This Item
经验公式(希尔记法):
C20H12Br2O2
CAS号:
分子量:
444.12
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
solid
mp
256-260 °C
InChI
1S/C20H12Br2O2/c21-15-9-11-5-1-3-7-13(11)17(19(15)23)18-14-8-4-2-6-12(14)10-16(22)20(18)24/h1-10,23-24H
InChI 密鑰
BRTBEAXHUYEXSY-UHFFFAOYSA-N
應用
(R)-(+)-3,3′-Dibromo-1,1′-bi-2-naphthol reacts with zirconium(IV) tert-butoxide to form a chiral zirconium complex, which can efficiently catalyze:
- anti-selective catalytic asymmetric aldol reactions of silyl enol ethers with aldehydes
- asymmetric intramolecular [3+2] cycloaddition of hydrazone/olefins
訊號詞
Warning
危險聲明
防範說明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Highly anti-selective catalytic asymmetric aldol reactions.
Ishitani H, et al.
Journal of the American Chemical Society, 122(22), 5403-5404 (2000)
Asymmetric intramolecular [3+2] cycloaddition reactions of acylhydrazones/olefins using a chiral zirconium catalyst.
Kobayashi S, et al.
Journal of the American Chemical Society, 124(46), 13678-13679 (2002)
商品
我们展示了一篇有关BINOL及其衍生物的文章。
We present an article concerning BINOL and Derivatives.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门