T1912
Paclitaxel
from Taxus yannanensis, powder
About This Item
Polecane produkty
pochodzenie biologiczne
Taxus yannanensis
Poziom jakości
Postać
powder
kolor
white
mp
213 °C (dec.) (lit.)
rozpuszczalność
DMSO: 50 mg/mL (can be stored frozen for several months)
acetonitrile: soluble
ethanol: soluble
methanol: soluble (undergoes transesterification)
spektrum działania antybiotyku
neoplastics
Tryb działania
DNA synthesis | interferes
inicjator
Bristol-Myers Squibb
temp. przechowywania
2-8°C
ciąg SMILES
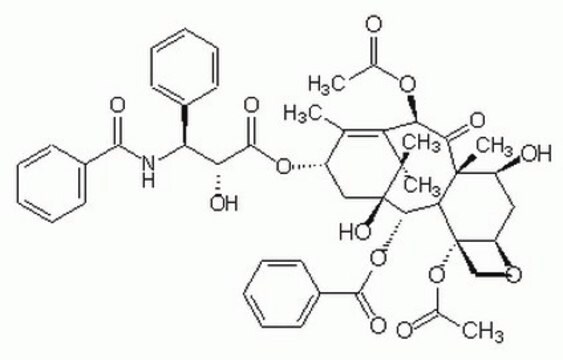
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)c6ccccc6)c7ccccc7
InChI
1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
Klucz InChI
RCINICONZNJXQF-MZXODVADSA-N
informacje o genach
human ... BCL2(596) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Cechy i korzyści
Przestroga
Uwaga dotycząca przygotowania
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Muta. 2 - Repr. 1B - STOT RE 1
Organy docelowe
Central nervous system,Bone marrow,Cardio-vascular system
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Discover Bioactive Small Molecules for ADME/Tox
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej