SAB1300492
Anti-PTPβ (C-term) antibody produced in rabbit
IgG fraction of antiserum, buffered aqueous solution
About This Item
Polecane produkty
pochodzenie biologiczne
rabbit
białko sprzężone
unconjugated
forma przeciwciała
IgG fraction of antiserum
rodzaj przeciwciała
primary antibodies
klon
polyclonal
Postać
buffered aqueous solution
reaktywność gatunkowa
human
metody
indirect ELISA: 1:1000
numer dostępu NCBI
numer dostępu UniProt
Warunki transportu
dry ice
temp. przechowywania
−20°C
docelowa modyfikacja potranslacyjna
unmodified
informacje o genach
human ... PTPRB(5787)
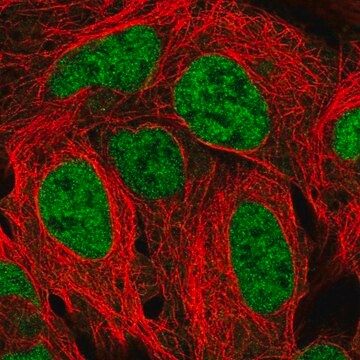
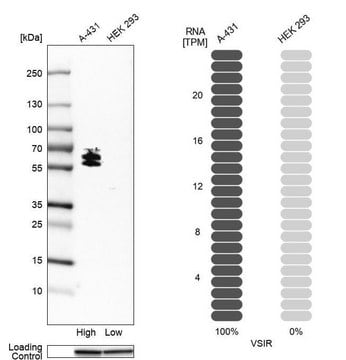
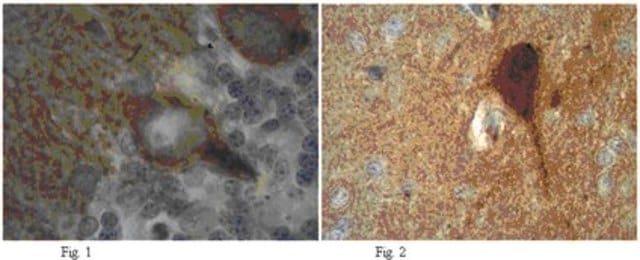
Opis ogólny
Immunogen
This antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide selected from the C-terminal region of human PTPbeta.
Działania biochem./fizjol.
Postać fizyczna
Not finding the right product?
Try our Narzędzie selektora produktów.
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
nwg
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej