C5483
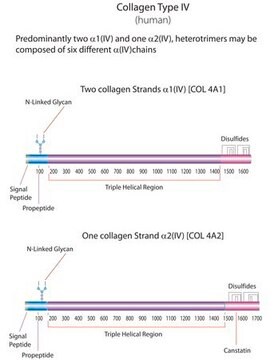
Human Collagen Type I
from human placenta, powder, ~95% (SDS-PAGE), suitable for cell culture
About This Item
Polecane produkty
product name
Collagen human, Bornstein and Traub Type I, acid soluble, powder, ~95% (SDS-PAGE)
pochodzenie biologiczne
human
Poziom jakości
Próba
~95% (SDS-PAGE)
Postać
powder
metody
cell culture | stem cell: suitable
rozpuszczalność
aqueous acid: ≤5 mg/mL
numer dostępu UniProt
temp. przechowywania
2-8°C
informacje o genach
human ... COL1A2(1278)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Collagen Type I has been used as a scaffold for the growth in vitro of stem cells in a wide variety of biomaterial engineering studies.
- as a component of extracellular matrix in the chemotaxis assay of the rat adipose-derived stem cells
- in adhesion assay of the adult retinal pigmented epithelium-19 (ARPE-19) cell line
- in the glycation aggregation and adsorption studies as a model system for arthritis
Działania biochem./fizjol.
Uwaga dotycząca przygotowania
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej