Wszystkie zdjęcia(1)
Key Documents
About This Item
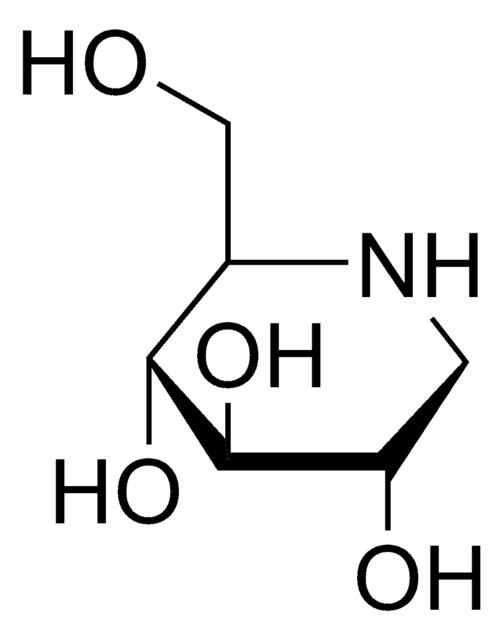
Wzór empiryczny (zapis Hilla):
C7H15NO5
Numer CAS:
Masa cząsteczkowa:
193.20
Kod UNSPSC:
12352201
Identyfikator substancji w PubChem:
NACRES:
NA.25
Polecane produkty
Próba
≥98.0% (TLC)
ciąg SMILES
OC[C@H]1N[C@H](CO)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5-,6+,7+/m1/s1
Klucz InChI
CLVUFWXGNIFGNC-OVHBTUCOSA-N
Zastosowanie
α-Homonojirimycin (HMJ) is used as an inhibitor of several carbohydrate degrading enzymes including α-glucosidases, glycoprotein processing enzyme glucosidase II and maltase.
Działania biochem./fizjol.
α-Homonojirimycin is a potent inhibitor of a range of α-glucosidases, as well as an inhibitor of the glycoprotein processing enzyme glucosidase II.
Opakowanie
Bottomless glass bottle. Contents are inside inserted fused cone.
Inne uwagi
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
This page may contain text that has been machine translated.
Kod klasy składowania
13 - Non Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
K Ikeda et al.
Carbohydrate research, 323(1-4), 73-80 (2000-04-27)
2,6-Dideoxy-7-O-(beta-D-glucopyranosyl) 2,6-imino-D-glycero-L-gulo- heptitol (7-O-beta-D-glucopyranosyl-alpha-homonojirimycin, 1) was isolated from the 50% methanol extract of the whole plant of Lobelia sessilifolia (Campanulaceae), which was found to potently inhibit rice alpha-glucosidase. Adenophorae radix, roots of Adenophora spp. (Campanulaceae), yielded new homonojirimycin derivatives, adenophorine
Gabriel M J Lenagh-Snow et al.
Organic letters, 14(8), 2050-2053 (2012-04-05)
Although there are 32 6-azidoheptitols, there are only 16 homonojirimycin (HNJ) stereoisomers. Two epimeric azidoalditols derived from d-mannose allow the synthesis in water of eight stereoisomers of HNJ.
O R Martin et al.
Bioorganic & medicinal chemistry letters, 9(21), 3171-3174 (1999-11-24)
The structure of a homonojirimycin isomer isolated from Aglaonema treublii and originally proposed as alpha-3,4-di-epi-homonojirimycin was revised to alpha-4-epi-homonojirimycin 3 ("alpha-homoallonojirimycin") on the basis of NMR analysis and synthetic studies. Its activity as a glycosidase inhibitor is compared to that
Shankar D Markad et al.
Bioorganic & medicinal chemistry, 14(16), 5535-5539 (2006-05-10)
Conjugate addition of n-butyl amine to d-glucose derived alpha,beta-unsaturated ester 4 afforded beta-amino esters 5a,b that on reduction of ester group, 1,2-acetonide deprotection, and reductive amination led to the formation of corresponding N-butyl 1-deoxy-D-gluco-homonojirimycin 2c and N-butyl 1-deoxy-L-ido-homonojirimycin 2d which
Chinami Kuriyama et al.
Bioorganic & medicinal chemistry, 16(15), 7330-7336 (2008-07-04)
We investigated in vitro inhibition of mammalian carbohydrate-degrading enzymes by six-membered sugar mimics and their evaluation in cell cultures. 1-Deoxynojirimycin (DNJ) showed no significant inhibition toward glycogen phosphorylase (GP) but was a potent inhibitor of another glycogen-degrading enzyme, amylo-1,6-glucosidase (1,6-GL)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej