669032
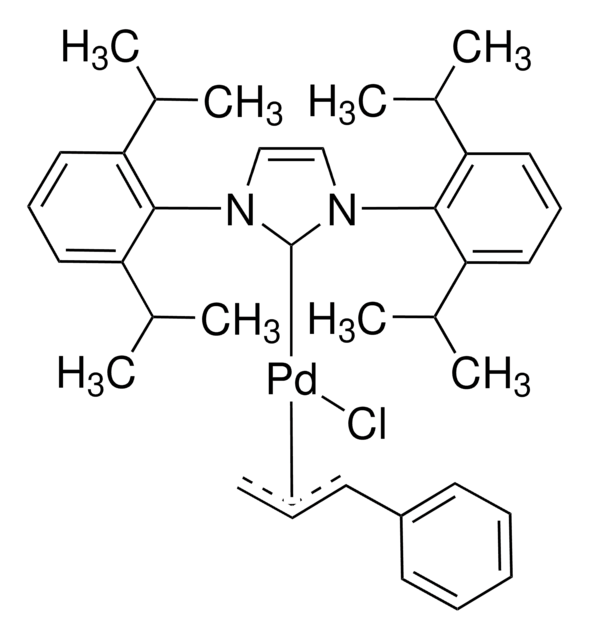
PEPPSI™-IPr catalyst
98%, Umicore
Synonim(y):
[1,3-Bis(2,6-Diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride
About This Item
Polecane produkty
Poziom jakości
Próba
98%
przydatność reakcji
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
producent / nazwa handlowa
Umicore
mp
230 °C
ciąg SMILES
Cl[Pd]Cl.Clc1cccnc1.CC(C)c2cccc(C(C)C)c2N3CN(C=C3)c4c(cccc4C(C)C)C(C)C
InChI
1S/C27H38N2.C5H4ClN.2ClH.Pd/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;6-5-2-1-3-7-4-5;;;/h9-16,18-21H,17H2,1-8H3;1-4H;2*1H;/q;;;;+2/p-2
Klucz InChI
BLDKGTGQENJFON-UHFFFAOYSA-L
Powiązane kategorie
Opis ogólny
Zastosowanie
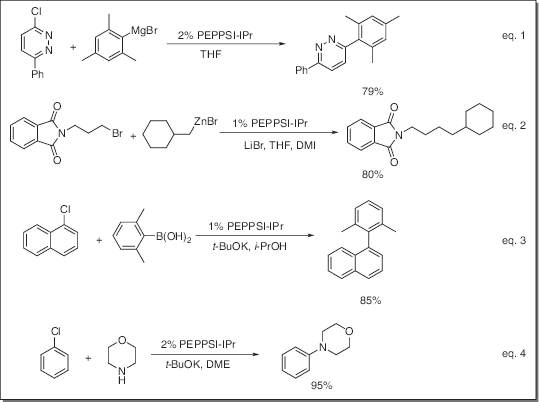
- Catalyst for Kumada-Tamao-Corriu (KTC) reaction (eq. 1)
- Catalyst for Negishi coupling reaction (eq. 2)
- Catalyst for Suzuki coupling reaction (eq. 3)
- Catalyst for Buchwald-Hartwig amination reaction (eq. 4)
For small scale and high throughput uses, product is also available as ChemBeads (931063)
Informacje prawne
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
Patented, U.S. Pat. No. 7,250,510. Sold under an exclusive license from Total Synthesis Ltd.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
Choose Ascentis® Express Peptide ES-C18 U/HPLC Columns based on Fused-Core® technology for fast and efficient separation of high-molecular weight compounds, such as peptides and small proteins. With a 2.7 µm particle size and rigorous testing, these columns offer reliable results and high efficiency.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)





