Yes, stoichiometric chemistry will be an appropriate method. The molecular weight can be used to calculate clindamycin in clindamycin phosphate. See the link below to review a sample Certificate of Analysis. Page 2 reports the purity of clindamycin as well as clindamycin phosphate.
https://www.sigmaaldrich.com/certificates/Graphics/COfAInfo/fluka/pdf/rtc/PHR1021_LRAD2296.pdf
推荐产品
等级
certified reference material
pharmaceutical secondary standard
质量水平
Agency
traceable to Ph. Eur. C2269000
traceable to USP 1138008
API类
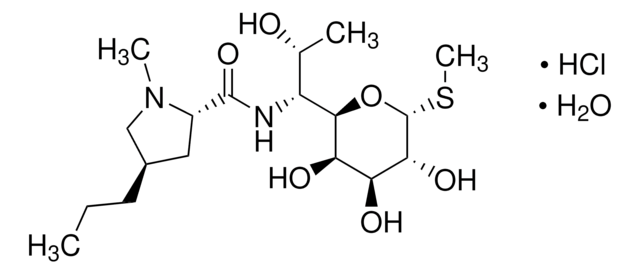
clindamycin
CofA
current certificate can be downloaded
技术
HPLC: suitable
gas chromatography (GC): suitable
应用
pharmaceutical (small molecule)
包装形式
neat
储存温度
-10 to -25°C
SMILES字符串
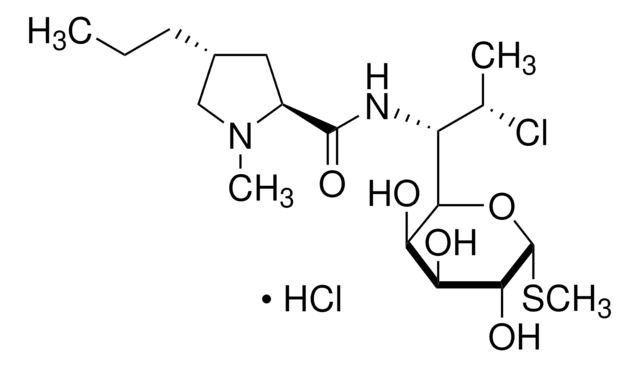
CCC[C@@H]1C[C@H](N(C)C1)C(=O)NC([C@H](C)Cl)C2O[C@H](SC)[C@H](OP(O)(O)=O)[C@@H](O)[C@H]2O
InChI
1S/C18H34ClN2O8PS/c1-5-6-10-7-11(21(3)8-10)17(24)20-12(9(2)19)15-13(22)14(23)16(18(28-15)31-4)29-30(25,26)27/h9-16,18,22-23H,5-8H2,1-4H3,(H,20,24)(H2,25,26,27)/t9-,10+,11-,12?,13+,14-,15?,16+,18+/m0/s1
InChI key
UFUVLHLTWXBHGZ-MWBQRTRKSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
Clindamycin is a common antibiotic drug generally employed for the treatment of infections caused by gram-positive aerobes and both gram-negative and gram-positive anaerobic pathogens.[1]
应用
生化/生理作用
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
其他说明
分析说明
附注
相关产品
警示用语:
Warning
危险分类
Acute Tox. 4 Oral - Eye Irrit. 2 - Lact. - Skin Sens. 1
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
其他客户在看
-
Hello, Which factor can be used to calculate Clindamycine base in a product having a reference this standar PHR1021 Clindamycine Phosphate? Can I use the stoichiometric factor 424.98/504.96?
1 answer-
Helpful?
-
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门