所有图片(1)
About This Item
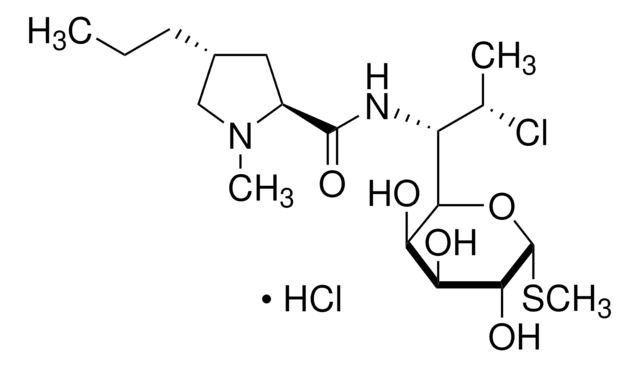
经验公式(希尔记法):
C18H34ClN2O8PS
CAS号:
分子量:
504.96
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
clindamycin
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
−20°C
SMILES 字串
CCC[C@@H]1C[C@H](N(C)C1)C(=O)NC([C@H](C)Cl)C2O[C@H](SC)[C@H](OP(O)(O)=O)[C@@H](O)[C@H]2O
InChI
1S/C18H34ClN2O8PS/c1-5-6-10-7-11(21(3)8-10)17(24)20-12(9(2)19)15-13(22)14(23)16(18(28-15)31-4)29-30(25,26)27/h9-16,18,22-23H,5-8H2,1-4H3,(H,20,24)(H2,25,26,27)/t9-,10+,11-,12?,13+,14-,15?,16+,18+/m0/s1
InChI 密鑰
UFUVLHLTWXBHGZ-MWBQRTRKSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Clindamycin is a lincosamide antibiotic which is considered as a semisynthetic derivative of lincomycin. It is mainly used as an antimicrobial agent.
應用
Clindamycin phosphate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Clindamycin Phosphate Gel
- Clindamycin Phosphate Vaginal Cream
- Clindamycin Phosphate Topical Suspension
- Clindamycin Phosphate Vaginal Inserts
- Clindamycin Phosphate Topical Solution
- Clindamycin Injection
生化/生理作用
Spectrum of Activity: Gram positive cocci and taxoplasma. Especially active against anaerobic bacteria.
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
其他說明
Antibacterial and antiprotozoal antibiotic of the licosamide class.
Sales restrictions may apply.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Lact. - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
D W Scott et al.
The Canadian veterinary journal = La revue veterinaire canadienne, 39(12), 753-756 (1998-12-23)
Clindamycin hydrochloride capsules (11 mg/kg body weight, q24 h) were administered orally to 20 dogs with deep staphylococcal pyoderma. Response to therapy was excellent in 100% of the dogs. Duration of therapy varied from 21 to 91 d, with an
Fulminant pseudomembranous colitis caused by clindamycin phosphate vaginal cream.
M F Trexler et al.
The American journal of gastroenterology, 92(11), 2112-2113 (1997-11-15)
James Q Del Rosso et al.
Cutis, 81(5), 405-408 (2008-06-12)
An aqueous gel formulation containing solubilized clindamycin phosphate 1.2% and a stable combination of both solubilized and crystalline tretinoin 0.025% (clin/tret) has been evaluated in 3 pivotal phase 3 studies, among other studies including a 52-week trial. The pivotal studies
Vuk Uskoković et al.
Journal of pharmaceutical sciences, 103(2), 567-579 (2014-01-03)
Nanoparticulate composites of hydroxyapatite (HAp) and chitosan were synthesized by ultrasound-assisted sequential precipitation and characterized for their microstructure at the atomic scale, surface charge, drug release properties, and combined antibacterial and osteogenic response. Crystallinity of HAp nanoparticles was reduced because
Le Ngoc Tran et al.
Journal of chromatography. A, 1356, 289-293 (2014-07-20)
An organic-inorganic silica/zirconia hybrid monolithic capillary column was prepared by sol-gel process in a fused-silica capillary by using triethoxysilylpropylcarbamate (TEOSPC) derivative of clindamycin phosphate (CLIP) as a chiral selector. A sol solution consisting of 6 × 10(-3)M of polyethylene glycol
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门