所有图片(1)
About This Item
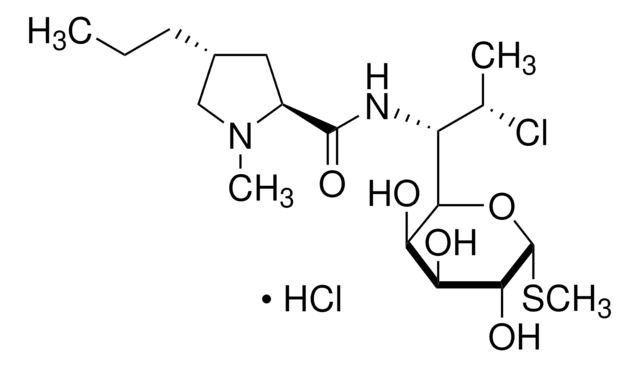
经验公式(希尔记法):
C18H34ClN2O8PS
CAS号:
分子量:
504.96
EC號碼:
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
clindamycin
製造商/商標名
EDQM
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
CCC[C@@H]1C[C@H](N(C)C1)C(=O)NC([C@H](C)Cl)C2O[C@H](SC)[C@H](OP(O)(O)=O)[C@@H](O)[C@H]2O
InChI
1S/C18H34ClN2O8PS/c1-5-6-10-7-11(21(3)8-10)17(24)20-12(9(2)19)15-13(22)14(23)16(18(28-15)31-4)29-30(25,26)27/h9-16,18,22-23H,5-8H2,1-4H3,(H,20,24)(H2,25,26,27)/t9-,10+,11-,12?,13+,14-,15?,16+,18+/m0/s1
InChI 密鑰
UFUVLHLTWXBHGZ-MWBQRTRKSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Clindamycin phosphate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Spectrum of Activity: Gram positive cocci and taxoplasma. Especially active against anaerobic bacteria.
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Antibacterial and antiprotozoal antibiotic of the licosamide class.
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Lact. - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Lawrence F Eichenfield et al.
Pediatric dermatology, 26(3), 257-261 (2009-08-27)
Treatments for mild to moderately severe acne usually combine retinoid and antimicrobial therapy. Recently, the US FDA approved the combination of 1.2% clindamycin (CLIN) and 0.025% tretinoin (RA) in a novel gel formulation for the treatment of mild to moderate
M Badawy Abdel-Naser et al.
Expert opinion on pharmacotherapy, 9(16), 2931-2937 (2008-10-22)
Clindamycin phosphate 1.2% together with tretinoin 0.025% as a gel (CTG) is a topical formulation of a fixed and stable combination approved by the FDA for the treatment of acne vulgaris in patients 12 years of age or older. The
Diane Thiboutot et al.
Journal of the American Academy of Dermatology, 59(5), 792-800 (2008-09-23)
We sought to evaluate efficacy, safety, and tolerability of a combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% (clindamycin-BPO 2.5%) aqueous gel in moderate to severe acne vulgaris. A total of 2813 patients, aged 12 years or older, were
Azadeh Ghaffari et al.
International wound journal, 9(2), 221-229 (2011-12-08)
Ethanol that affects hydration of skin and used in wound treatment formulations was studied here for its effect on permeation of drugs through burn eschar and to investigate the presence of a porous pathway in this barrier. In this study
Asuka Munehiro et al.
The Journal of dermatological treatment, 23(3), 172-176 (2011-07-16)
Cosmetic moisturisers were applied to one side of the face of 18 male Japanese patients with acne vulgaris who were treated with a topical administration of adapalene and clindamycin phosphate gels. We assessed the alleviating effect of the moisturisers on
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门