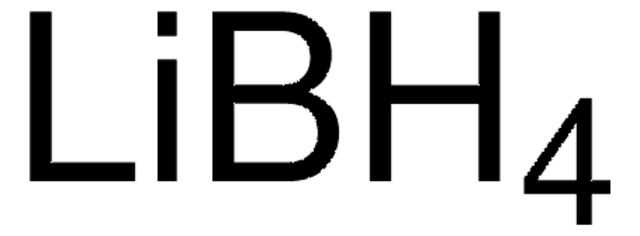推荐产品
表单
liquid
反应适用性
reagent type: reductant
浓度
0.5 M in diethyl ether
密度
0.719 g/mL at 25 °C
SMILES字符串
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI key
UUKMSDRCXNLYOO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
应用
反应物用于:
- 镓、铟、铼和锌三(巯基咪唑基)氢硼酸配合物的制备
- 机械化学复分解反应
- 非催化水解制氢
- 大型金单层保护簇的生长
- 阴离子取代反应
- 脱氢反应
Lithium borohydride is a commonly used reducing agent. It can also be used a reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes.
- Mechano-chemical metathesis reactions.
- Noncatalytic hydrolysis for hydrogen generation.
- Growth of large gold monolayer protected-clusters.
- Anion substitution reactions.
- Dehydrogenation reactions.
其他说明
Material may form precipitate on standing, which does not affect its use.
警示用语:
Danger
危险分类
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
靶器官
Central nervous system
补充剂危害
储存分类代码
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
闪点(°F)
-40.0 °F
闪点(°C)
-40 °C
个人防护装备
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Controlled growth and catalytic activity of gold monolayer protected clusters in presence of borohydride salts.
Dasog M, et al.
Chemical Communications (Cambridge, England), 47(30), 8569-8571 (2011)
Effect of hydrogen back pressure on dehydrogenation behavior of LiBH4-based reactive hydride composites.
Shim J H, et al.
The Journal of Physical Chemistry Letters, 1(1), 59-63 (2009)
Lithium Borohydride.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2009)
Synthesis, crystal structure, and thermal properties of the first mixed-metal and anion-substituted rare earth borohydride LiCe (BH4) 3Cl.
Frommen c, et al.
The Journal of Physical Chemistry, 115(47), 23591-23602 (2011)
Mechano-chemical reactions in LiBH4+ VCln (n= 2 and 3) mixtures.
Llamas-Jansa I, et al.
J. Alloy Compounds, 509, S684-S687 (2011)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门