推荐产品
一般說明
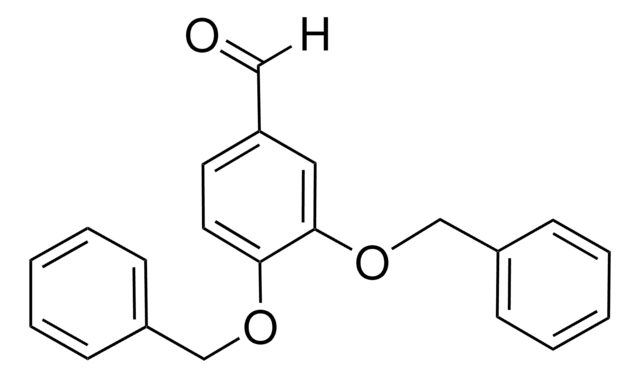
4-Benzyloxy-3-methoxybenzaldehyde reacts with benzohydrazide to yield (E)-N′-(4-benzyloxy-3-methoxybenzylidene)benzohydrazide.
應用
4-Benzyloxy-3-methoxybenzaldehyde was used in the synthesis of 1,2-bis(4-benzyloxy-3-methoxyphenyl)-3-hydroxy-propionic acid. It was also used in first enantioselective total synthesis of a neurotrophic (-)-talaumidin.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Synthesis of the erythro and threo forms of 1, 2-bis (4-hydroxy-3-methoxyphenyl)-l, 3-propanediol.
Berndtsson L, et al.
Acta Chemica Scandinavica. Series B, 34, 453-455 (1980)
(E)-N'-(4-Benzyloxy-3-methoxybenzylidene) benzohydrazide.
He Y-Z and Liu D-Z.
Acta Crystallographica Section E, Structure Reports Online, 61(11), o3855-o3856 (2005)
First enantioselective synthesis of (-)-talaumidin, a neurotrophic diaryltetrahydrofuran-type lignan.
Esumi T, et al.
Tetrahedron Letters, 47(24), 3979-3983 (2006)
C A Jackson et al.
Journal of applied microbiology, 122(4), 940-952 (2017-01-17)
The aim of this work was to isolate novel lignin-degrading organisms. Several pure cultures of bacteria that degrade lignin were isolated from bacterial consortia developed from decaying biomass. Among the isolates, Rhizobium sp. strain YS-1r (closest relative of Rhizobium petrolearium
Alla V Lipeeva et al.
European journal of medicinal chemistry, 100, 119-128 (2015-06-17)
A series of 2-(4-R-triazolyl)substituted 3-oxo-2,3-dihydrofurocoumarins have been synthesized by a regioselective cycloaddition of 2-azidooreoselone 1 or 2-azido-9-[(4-methylpiperazin-1-yl)methyl]oreoselone 2 with various alkynes in the presence of Cu(II)/ascorbate in water/methylene chloride reaction medium. The structure of 2-azidooreoselone was established by X-ray structure
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门