Alle Fotos(1)
Wichtige Dokumente
535087
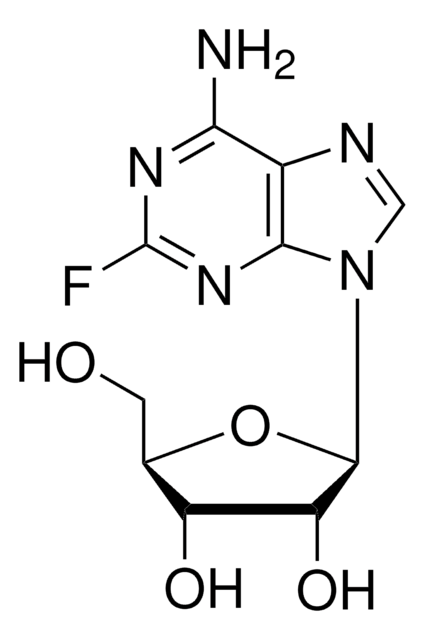
2-Fluoradenin
96%
Synonym(e):
2-Fluor-7(9)H-purin-6-ylamin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H4FN5
CAS-Nummer:
Molekulargewicht:
153.12
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
96%
mp (Schmelzpunkt)
>350 °C (lit.)
Funktionelle Gruppe
fluoro
SMILES String
Nc1[nH]c(F)nc2ncnc12
InChI
1S/C5H4FN5/c6-5-10-3(7)2-4(11-5)9-1-8-2/h1H,(H3,7,8,9,10,11)
InChIKey
WKMPTBDYDNUJLF-UHFFFAOYSA-N
Verwandte Kategorien
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Yukio Kitade et al.
Nucleic acids research. Supplement (2001), (3)(3), 5-6 (2003-09-27)
Carbocyclic and acyclic nucleosides possessing 2-fluoroadenine, such as 2-fluoronoraristeromycin (6) and 2-fluoro-9-[(2S,3R)-2,3,4-trihydroxy-butyl-1-yl]adenine (8), were synthesized and their inhibitory activities against human and Plasmodium falciparum recombinant SAH hydrolase were investigated.
P Huang et al.
Biochemical pharmacology, 36(18), 2945-2950 (1987-09-15)
2-Fluoroadenine (F-Ade) is a metabolite of 9-beta-D-arabinofuranosyl-2-fluoroadenine (F-ara-A) that may be involved in the development of toxic side effects from this anticancer drug. The liberation of F-Ade from F-ara-A has been examined in different biological systems. Extracts of Escherichia coli
D Voeks et al.
Gene therapy, 9(12), 759-768 (2002-06-01)
A gene-directed enzyme pro-drug therapy (GDEPT) based on purine nucleoside phosphorylase (PNP), that converts the prodrug, fludarabine to 2-fluoroadenine, has been described, but studies are limited compared with other GDEPTs. We investigated the in vitro and in vivo efficacies of
X Y Wang et al.
Gene therapy, 11(21), 1559-1567 (2004-09-03)
Gene-directed enzyme prodrug therapy (GDEPT) based on the Escherichia coli enzyme, purine nucleoside phosphorylase (PNP), provides a novel strategy for treating slowly growing tumors like prostate cancer (CaP). PNP converts systemically administered prodrug, fludarabine phosphate, to a toxic metabolite, 2-fluoroadenine
B E Miller et al.
Cancer research, 46(1), 89-93 (1986-01-01)
A series of subpopulation lines derived from a single mouse mammary tumor were tested for their ability to interact with each other in metabolic cooperation assays in three-dimensional collagen gel cultures. Inhibition of growth in the presence of selective drug
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.