추천 제품
분석
≥98% (HPLC)
형태
powder
색상
yellow to orange
solubility
DMSO: 5 mg/mL, clear
저장 온도
2-8°C
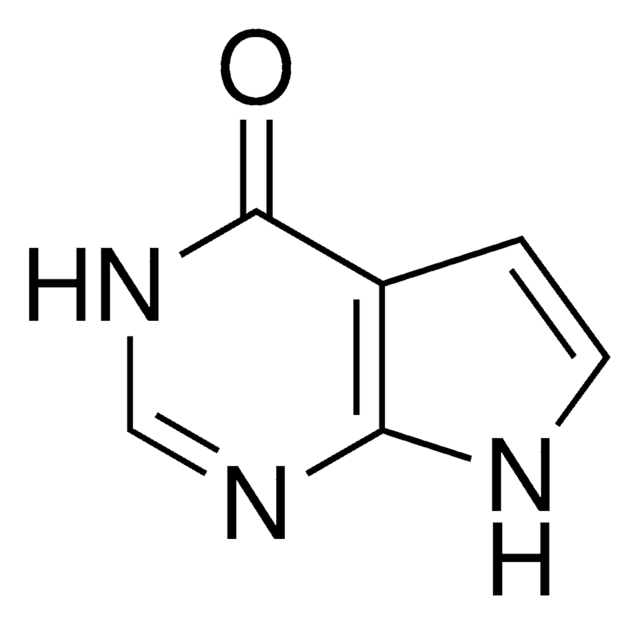
SMILES string
[I-].CC[n+]1c(\C=C\N(C)c2ccccc2)n(-c3ccccc3)c4ccc(cc14)-c5nc6ccccc6s5
InChI
1S/C31H27N4S.HI/c1-3-34-28-22-23(31-32-26-16-10-11-17-29(26)36-31)18-19-27(28)35(25-14-8-5-9-15-25)30(34)20-21-33(2)24-12-6-4-7-13-24;/h4-22H,3H2,1-2H3;1H/q+1;/p-1
InChI key
NAYRELMNTQSBIN-UHFFFAOYSA-M
생화학적/생리학적 작용
5-(2-Benzothiazolyl)-3-ethyl-2-[2-(methylphenylamino)-ethenyl]-1-phenyl-1H-benzimidazolium, or Akt Inhibitor IV, is a cell-permeable benzimidazole compound that inhibits Akt phosphorylation/activation by targeting the ATP binding site of a kinase upstream of Akt, but downstream of PI3K. Shown to block Akt-mediated FOXO1a nuclear export (IC50 = 0.625μM) and cell proliferation (IC50 < 1.25μM) in 786-O cells. Unlike phosphatidylinositol analog-based Akt inhibitors, this inhibitor does not affect PI3K.
특징 및 장점
This compound is featured on the PKB/Akt page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Jinxia Liu et al.
Oncogenesis, 9(9), 84-84 (2020-09-26)
β-Adrenergic receptor (β-AR) signalling is strongly associated with tumour progression by the coupling of β-ARs with either a G protein or β-arrestin; however, the related mechanism underlying hepatocellular carcinoma (HCC) metastasis is not clear. Here, we reveal that the transcription
Emmanuel Chautard et al.
Neuro-oncology, 12(5), 434-443 (2010-04-22)
Radiation therapy plays a central role in the treatment of glioblastoma, but it is not curative due to the high tumor radioresistance. Phosphatidyl-inositol 3-kinase/protein kinase B (Akt) and Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathways serve
Wenda Di et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 34(2), 2075-2086 (2020-01-08)
In the free-living nematode Caenorhabditis elegans, the serine/threonine-specific protein kinase, AKT, is known to play a key role in dauer formation, life-span, and stress-resistance through the insulin-like signaling pathway. Although the structure and function of AKT-coding genes of C. elegans
Kenji Hayata et al.
Journal of pharmacological sciences, 108(3), 348-354 (2008-11-15)
To obtain compounds that promote glucose uptake in muscle cells, the novel cell lines A31-IS derived from Balb/c 3T3 A31 and C2C12-IS from mouse myoblast C2C12 were established. In both cell lines, glucose consumption was induced by insulin and suppressed
Pavithra Lakshminarasimhan Chavali et al.
The Journal of biological chemistry, 286(11), 9393-9404 (2010-12-08)
Hypoxia promotes neural stem cell proliferation, the mechanism of which is poorly understood. Here, we have identified the nuclear orphan receptor TLX as a mediator for proliferation and pluripotency of neural progenitors upon hypoxia. We found an enhanced early protein
문서
We offer many products related to PKB/Akt for your research needs.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.