おすすめの製品
グレード
reagent grade
製品種目
Vetec™
アッセイ
98%
mp
232-234 °C (dec.) (lit.)
SMILES記法
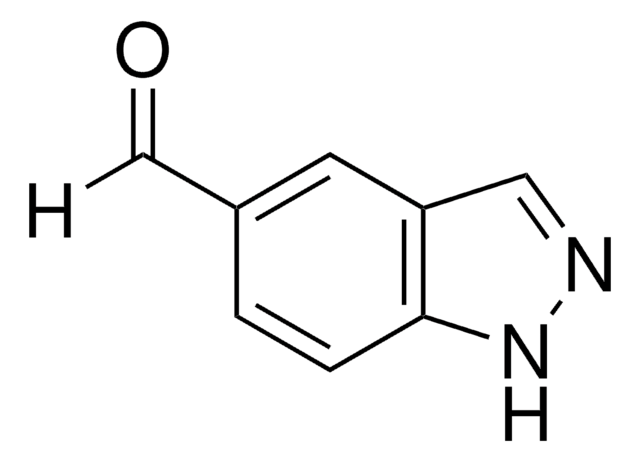
OC(=O)c1c[nH]c2ccccc12
InChI
1S/C9H7NO2/c11-9(12)7-5-10-8-4-2-1-3-6(7)8/h1-5,10H,(H,11,12)
InChI Key
KMAKOBLIOCQGJP-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
以下の調製のための反応剤:
- 抗癌剤。
- アミノ酸およびペプチドの誘導体。
- セロトニン5-HT4受容体アンタゴニスト。
- 一級アシル尿素。
- ヘッジホッグ経路におけるGli1媒介性転写の阻害剤。
- セロトニン5-HT6アンタゴニスト。
- 最晩期抗原-4(VLA-4)アンタゴニスト。
- EphB3受容体チロシンキナーゼ阻害剤。
- アルツハイマー病の治療薬の可能性。
- ビニルエステルシュードトリペプチドプロテアソーム阻害剤。
法的情報
Vetec is a trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Toyokazu Yoshida et al.
Bioscience, biotechnology, and biochemistry, 66(11), 2388-2394 (2003-01-01)
After enrichment culture with indole-3-carboxylate in static culture, a novel reversible decarboxylase, indole-3-carboxylate decarboxylase, was found in Arthrobacter nicotianae FI1612 and several molds. The enzyme reaction was examined in resting-cell reactions with A. nicotianae FI1612. The enzyme activity was induced
J J Michnovicz et al.
Journal of the National Cancer Institute, 82(11), 947-949 (1990-06-06)
Dietary indoles in cruciferous vegetables induce cytochrome P450 enzymes and have prevented tumors in various animal models. Because estradiol metabolism is also cytochrome P450 mediated and linked to breast cancer risk, indoles may similarly reduce estrogen-responsive tumors in humans. We
Paweł Bednarek
Current opinion in plant biology, 15(4), 407-414 (2012-03-27)
In plants, a host's responses to an attempted infection include activation of various secondary metabolite pathways, some of which are specific for particular plant phylogenetic clades. Phytochemicals that represent respective end products in plant immunity have been stereotypically linked to
Mu-Yang Wang et al.
Journal of integrative plant biology, 54(7), 471-485 (2012-05-26)
Camalexin (3-thiazol-2'-yl-indole) is the major phytoalexin found in Arabidopsis thaliana. Several key intermediates and corresponding enzymes have been identified in camalexin biosynthesis through mutant screening and biochemical experiments. Camalexin is formed when indole-3-acetonitrile (IAN) is catalyzed by the cytochrome P450
M U Ahmad et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 23(9), 841-847 (1985-09-01)
The nitrosation of gramine, a tertiary amine alkaloid present in barley malt, was carried out by reaction with sodium nitrite in buffered acetic acid (pH 3.4) for 1 hr at room temperature. Two major non-volatile products of the nitrosation reaction
アクティブなフィルタ
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)