おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
powder
アプリケーション
metabolomics
vitamins, nutraceuticals, and natural products
保管温度
−20°C
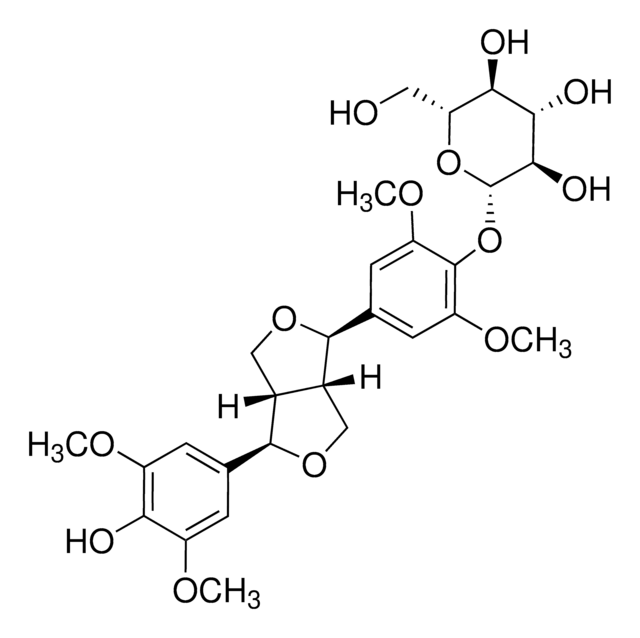
SMILES記法
O[C@H]1[C@H](O)[C@@H](CO)O[C@@H](OC2=CC=C([C@H]3OC[C@@]4([H])[C@]3([H])CO[C@@H]4C5=CC=C(O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@H]6O)C(OC)=C5)C=C2OC)[C@@H]1O
InChI
1S/C32H42O16/c1-41-19-7-13(3-5-17(19)45-31-27(39)25(37)23(35)21(9-33)47-31)29-15-11-44-30(16(15)12-43-29)14-4-6-18(20(8-14)42-2)46-32-28(40)26(38)24(36)22(10-34)48-32/h3-8,15-16,21-40H,9-12H2,1-2H3/t15-,16-,21+,22+,23+,24+,25-,26-,27+,28+,29+,30+,31+,32+/m0/s1
InChI Key
ZJSJQWDXAYNLNS-FUPWJLLWSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
Pinoresinol diglucoside (PDG) is an important lignan found in the bark of the traditional Chinese medicinal plant Eucommia ulmoides, also known as Tu-Chung. It is also associated with other plants such as Forsythia suspensa and Styrax spp. In plants, PDG is produced through the phenylpropanoid pathway.
Pinoresinol diglucoside is an important lignan found in the bark of the Eucommia ulmoides, which is known in traditional Chinese medicine as du zhong. In plants, pinoresinol diglucoside is produced through the phenylpropanoid pathway.
生物化学的/生理学的作用
Pinoresinol diglucoside has antihypertensive properties. It serves as a α-glucosidase inhibitor. α-Glucosidase catalyzes the conversion of carbohydrates to glucose in the small intestine. Hence, pinoresinol diglucoside might be useful as an antidiabetic agent.
Pinoresinol diglucoside is a potent antihypertensive, anti-inflammatory and antioxidant agent. It also exerts tumor-suppressive and anti-osteoporotic properties. PDG serves as an α-glucosidase inhibitor that catalyzes the conversion of carbohydrates to glucose in the small intestine. Hence, it may serve as an antidiabetic agent in the management of diabetes. It displays neuroprotective properties by alleviating ischemic stroke-related brain injury in mice model.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Bioconversion of pinoresinol diglucoside and pinoresinol from substrates in the phenylpropanoid pathway by resting cells of Phomopsis sp. XP-8
Zhang Y, et al.
PLoS ONE, 10(9), e0137066-e0137066 (2015)
J Lukl et al.
Vnitrni lekarstvi, 38(1), 1-5 (1992-01-01)
In the course of nine years the authors investigated in 3570 patients hospitalized on account of acute coronary syndrome associations between unstable angina pectoris (UAP) and a acute myocardial infarction (NMI). A total of 1732 patients were admitted with UAP
Pinoresinol diglucoside is screened as a putative ?-glucosidase inhibiting compound in Actinidia arguta leaves
Kwon D, et al.
Journal of the Korean Society for Applied Biological Chemistry, 57(4) (2014)
Zhenhong Gao et al.
Journal of microbiology and biotechnology, 27(8), 1428-1440 (2017-06-18)
Phomopsis sp. XP-8 (an endophytic fungus) was previously found to produce pinoresinol diglucoside (PDG), a major antihypertensive compound of Tu-Chung (the bark of Eucommia ulmoides Oliv.), which is widely used in Chinese traditional medicines. In the present study, two bioconversion
M Bulat et al.
The Journal of physiology, 275, 191-197 (1978-02-01)
1. After inhibition of 5-hydroxyindoleacetic acid (5-HIAA) transport by repeated administration of probenecid in cats, this acid accumulates linearly in the lumbosacral cord, lumbar cerebrospinal fluid (c.s.f.) and cisternal fluid during 5 hr of experiment. 2. A simple mathematical analysis
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)