おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
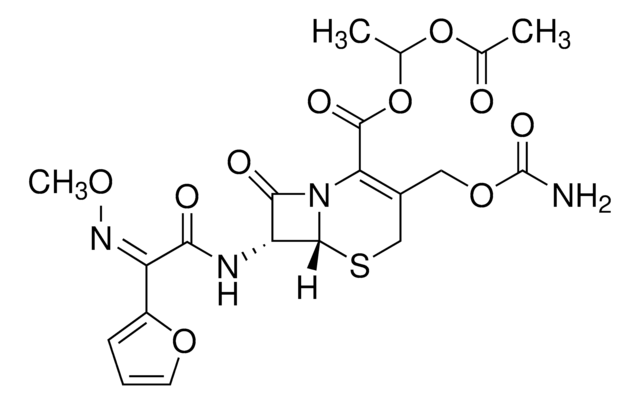
cefuroxime
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
[Na+].CO\N=C(/C(=O)N[C@H]1[C@H]2SCC(COC(N)=O)=C(N2C1=O)C([O-])=O)c3ccco3
InChI
1S/C16H16N4O8S.Na/c1-26-19-9(8-3-2-4-27-8)12(21)18-10-13(22)20-11(15(23)24)7(5-28-16(17)25)6-29-14(10)20;/h2-4,10,14H,5-6H2,1H3,(H2,17,25)(H,18,21)(H,23,24);/q;+1/p-1/b19-9-;/t10-,14-;/m1./s1
InChI Key
URDOHUPGIOGTKV-JTBFTWTJSA-M
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
アプリケーション
包装
その他情報
関連製品
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
C0695000-1EA:
C0695000:
Choose from one of the most recent versions:
試験成績書(COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact カスタマーサポート
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)