おすすめの製品
品質水準
アッセイ
≥95% (TLC)
形状
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
色
white
溶解性
DMSO: 25 mg/mL
輸送温度
ambient
保管温度
−20°C
InChI
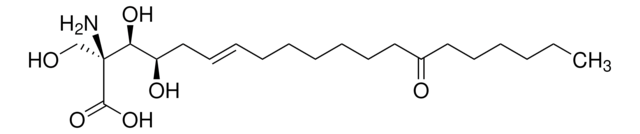
1S/C21H39NO6/c1-2-3-4-10-13-17(24)14-11-8-6-5-7-9-12-15-18(25)19(26)21(22,16-23)20(27)28/h9,12,18-19,23,25-26H,2-8,10-11,13-16,22H2,1H3,(H,27,28)/b12-9+/t18-,19+,21+/m1/s1
InChI Key
ZZIKIHCNFWXKDY-GNTQXERDSA-N
詳細
A potent immunosuppressant. Displays 10- to 100-fold more potent immunosuppressant activity than cyclosporin A. Potently inhibits serine palmitoyltransferase (SPT; Ki = 280 pM) thereby blocking the synthesis of ceramide. Disrupts substratum adhesion of melanoma cells. Inhibits cell proliferation and induces apoptosis in IL-2 dependent murine cytotoxic T lymphocyte cell line CTLL-2.
生物化学的/生理学的作用
Cell permeable: no
Primary Target
Serine palmitoyltransferase
Serine palmitoyltransferase
Product does not compete with ATP.
Reversible: no
Target Ki: 280 pM against serine palmitoyltransferase
警告
Toxicity: Harmful (C)
再構成
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
その他情報
Hanada, K., et al. 2000. Biochem. Pharmacol.59, 1211.
Chen, J.K., et al. 1999. Chem. Biol. 6, 221.
Hidari, K.I.P.J., et al. 1996. J. Biol. Chem. 271, 14636.
Nakamura, S., et al. 1996. J. Biol. Chem. 271, 1255.
Mikaye, Y., et al. 1995. Biochem. Biophys. Res. Commun. 211, 396.
Fujita, T., et al. 1994. J. Antibiot. 47, 208.
Chen, J.K., et al. 1999. Chem. Biol. 6, 221.
Hidari, K.I.P.J., et al. 1996. J. Biol. Chem. 271, 14636.
Nakamura, S., et al. 1996. J. Biol. Chem. 271, 1255.
Mikaye, Y., et al. 1995. Biochem. Biophys. Res. Commun. 211, 396.
Fujita, T., et al. 1994. J. Antibiot. 47, 208.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
476300-MG:
476300-1.1ML:
476300-5MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
T Fujita et al.
The Journal of antibiotics, 47(2), 208-215 (1994-02-01)
A potent immunosuppressive activity was found in the culture broth of the fungus Isaria sinclairii (ATCC 24400). The metabolite, ISP-I ((2S,3R,4R)-(E)-2-amino-3,4-dihydroxy-2- hydroxymethyl-14-oxoeicos-6-enoic acid, myriocin = thermozymocidin) suppressed the proliferation of lymphocytes in mouse allogeneic mixed lymphocyte reaction, but had no
Song Yu et al.
Foods (Basel, Switzerland), 12(13) (2023-07-14)
Human health is seriously threatened by mycotoxin contamination, yet health risk assessments are typically based on just one mycotoxin, potentially excluding the additive or competitive interactions between co-occurring mycotoxins. In this investigation, we evaluated the individual or combined toxicological effects
J K Chen et al.
Chemistry & biology, 6(4), 221-235 (1999-04-01)
Myriocin is a natural product that potently induces apoptosis of a murine cytotoxic T lymphocyte cell line (CTLL-2) and inhibits a serine palmitoyltransferase (SPT) activity that has been detected in cell extracts and is thought to initiate sphingolipid biosynthesis. Because
S Nakamura et al.
The Journal of biological chemistry, 271(3), 1255-1257 (1996-01-19)
In our previous study, the sphingosine-like immunosuppressant, ISP-1, was found to suppress the proliferation of an interleukin-2-dependent cytotoxic T cell line, CTLL-2, through the inhibition of serine palmitoyltransferase, which catalyzes the committed step of sphingolipid biosynthesis. Analysis of the effect
Y Miyake et al.
Biochemical and biophysical research communications, 211(2), 396-403 (1995-06-15)
ISP-1/myriocin is a new type of remarkably potent immunosuppressant, the structure of which is homologous to sphingosine. ISP-1/myriocin inhibited the proliferation of an IL-2-dependent mouse cytotoxic T cell line, CTLL-2, at nanomole concentrations. ISP-1/myriocin inhibits serine palmitoyltransferase activity at picomole
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)