すべての画像(1)
サイズを選択してください
表示を変更する
25 G
¥27,300
About This Item
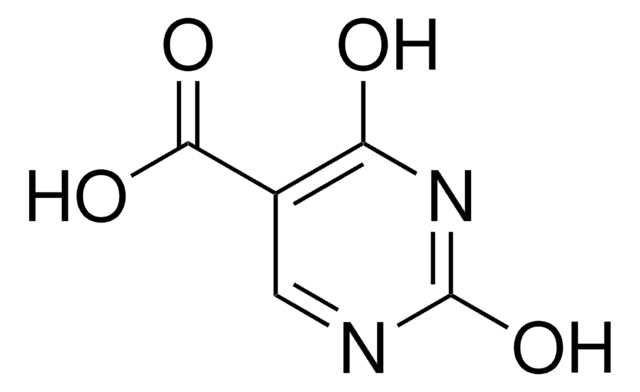
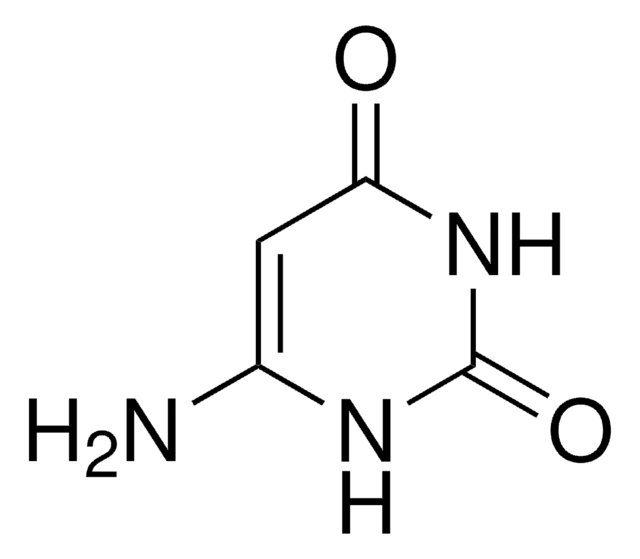
実験式(ヒル表記法):
C4H5N3O2
CAS番号:
分子量:
127.10
Beilstein:
127250
EC Number:
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
アッセイ
98%
フォーム
powder
mp
>300 °C (lit.)
SMILES記法
NC1=CNC(=O)NC1=O
InChI
1S/C4H5N3O2/c5-2-1-6-4(9)7-3(2)8/h1H,5H2,(H2,6,7,8,9)
InChI Key
BISHACNKZIBDFM-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
855286-VAR:
855286-25G:
855286-100G:
855286-BULK:
855286-5G:
Paulina Spisz et al.
International journal of molecular sciences, 21(17) (2020-09-05)
Hypoxia-a hallmark of solid tumors-dramatically impairs radiotherapy, one of the most common anticancer modalities. The adverse effect of the low-oxygen state can be eliminated by the concomitant use of a hypoxic cell radiosensitizer. In the present paper, we show that
Asmaa M Fahim et al.
Current computer-aided drug design, 16(4), 486-499 (2019-07-11)
In this investigation, 2-cyano-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) acetamide (3) reacts with dimethylformamide dimethyl acetal (DMF-DMA) to afford the corresponding (E)- 2-cyano-3-(dimethylamino)-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylam-ide (4) utilizing microwave irradiation. The condensation reactions of acrylamide derivative 4 with hydrazine derivatives obtain pyrazole derivatives 6a and 6b; respectively. The
D Suciu
The International journal of biochemistry, 23(11), 1245-1249 (1991-01-01)
1. The results of this study have contributed to the definition of three categories of chemical inhibitors of DNA replication in mammalian cells. 2. Inhibitors of replicon cluster initiation [4-nitroquinoline-N-oxide (4-NQO), etoposide (VP-16), teniposide (VM-26), amsacrine (m-AMSA), N-methyl-N'-nitro-N-nitrozoguanidine (MNNG), cis-Pt(II)diammine
A González-Fernández et al.
Mutation research, 149(2), 275-281 (1985-04-01)
Proliferating plant cells treated during the late S period with 5-aminouracil (AU), give the typical response that DNA-damaging agents induce, characterized by: an important mitotic delay, and a potentiation of the chromosome damage by caffeine post-treatment. The study of labelled
J E Thomas et al.
Cell and tissue kinetics, 16(3), 285-301 (1983-05-01)
The influence of 5-amino uracil (5-AU) was investigated on the cell cycle of log growth and division-synchronized Tetrahymena pyriformis GL. The division index of log growth phase Tetrahymena was suppressed by 50% after 40 min in 8 mM 5-AU. Cells
アクティブなフィルタ
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)