すべての画像(1)
サイズを選択してください
表示を変更する
500 MG
¥32,900
About This Item
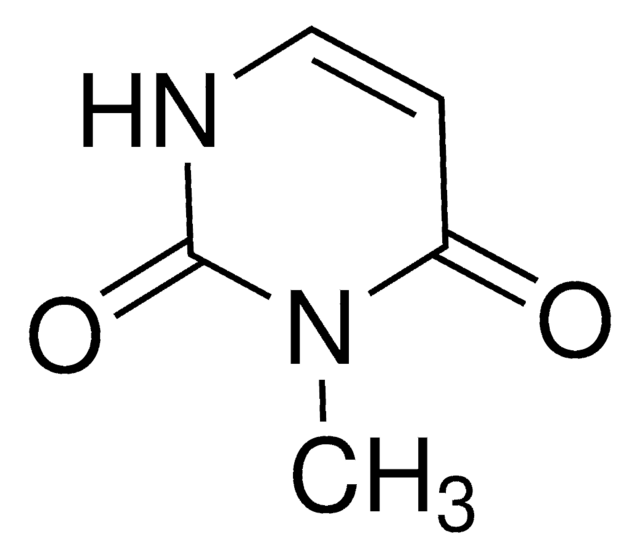
実験式(ヒル表記法):
C5H6N2O2
CAS番号:
分子量:
126.11
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
品質水準
アッセイ
99%
mp
236-238 °C (lit.)
溶解性
1 M NaOH: soluble 50 mg/mL, clear, colorless
SMILES記法
CN1C=CC(=O)NC1=O
InChI
1S/C5H6N2O2/c1-7-3-2-4(8)6-5(7)9/h2-3H,1H3,(H,6,8,9)
InChI Key
XBCXJKGHPABGSD-UHFFFAOYSA-N
関連するカテゴリー
詳細
1-Methyluracil is of special importance in biochemistry, since uracil attaches ribose in ribonucleic acid (RNA) just precisely at the N1 atom[1]. H-bond complex formation between 1-methyluracil and glycine has been investigated by theoretical calculations and FT-IR spectroscopy in Ar matrices[2]. It forms 1:1 complexes with 9-ethyl-8-bromo-2,6-diaminopurine and the complex structure has been determined by three-dimensional X-ray diffraction methods[3].
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Carc. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
293768-BULK:
293768-VAR:
293768-500MG:
293768-100MG:
Bram Boeckx et al.
The journal of physical chemistry. B, 116(39), 11890-11898 (2012-09-12)
The H-bond complex formation between 1-methyluracil and glycine has been investigated by theoretical calculations and the most stable complex configurations have been identified by FT-IR spectroscopy in Ar matrices. The importance of this H-bonding system is huge since all DNA
V I Poltev et al.
Journal of biomolecular structure & dynamics, 9(1), 101-111 (1991-08-01)
A number of nucleic acid base pairs and complexes between the bases and the amide group of acrylamide have been studied experimentally by using mass spectrometry and theoretically by the method of atom-atom potential function calculations. It has been found
V I Poltev et al.
Molekuliarnaia biologiia, 29(2), 365-375 (1995-03-01)
Monte Carlo simulation of hydration of keto and enol tautomers of 9-methylguanine (G) and 1-methyluracil (U) has been performed in relation to a possible role of tautomer transitions of DNA bases in mutagenesis. The comparison of the simulation results with
E Sagstuen et al.
Radiation research, 149(2), 120-127 (1998-02-11)
Single crystals of the co-crystalline complex of 1-methyluracil and 9-ethyladenine were X-irradiated and studied using EPR, ENDOR and FSE spectroscopic techniques at 10 K. All together seven radicals were identified, and experimental evidence for at least one more species, as
Base-pairing configurations between purines and pyrimidines in the solid state. V. Crystal and molecular structure of two 1:1 hydrogen-bonded complexes, 1-methyluracil: 9-ethyl-8-bromo-2,6-diaminopurine and 1-ethylthymine: 9-ethyl-8-bromo-2,6--diaminopurine.
G Simundza et al.
Journal of molecular biology, 48(2), 263-278 (1970-03-14)
アクティブなフィルタ
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)





![6-Methylthieno[2,3-d]pyrimidine-2,4(1H,3H)-dione](/deepweb/assets/sigmaaldrich/product/structures/393/943/c932f315-dd4b-4939-aea6-646238005e48/640/c932f315-dd4b-4939-aea6-646238005e48.png)


