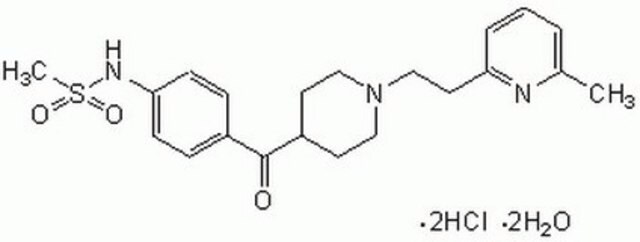
C5235
Cytochrome P450 human
4F3B isozyme microsomes, with P450 Reductase and cytochrome b5, recombinant, expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
About This Item
Prodotti consigliati
Origine biologica
human
Livello qualitativo
Ricombinante
expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
Forma fisica
solution
PM
45-60 kDa
Confezionamento
vial of 0.5 nmol
N° accesso UniProt
applicazioni
cell analysis
Condizioni di spedizione
dry ice
Temperatura di conservazione
−70°C
Informazioni sul gene
human ... CYP4F3(4051)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
Definizione di unità
Stato fisico
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.