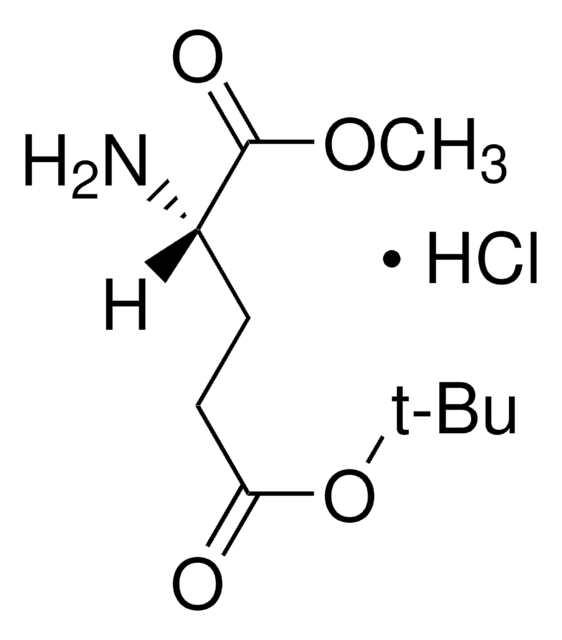
C3394
Cordycepin
from Cordyceps militaris, ≥98% (HPLC), powder, adenosine analogue
Sinonimo/i:
3′-Deoxyadenosine
About This Item
Prodotti consigliati
product name
Cordycepin, from Cordyceps militaris
Origine biologica
Cordyceps militaris
Livello qualitativo
Forma fisica
powder
Spettro attività antibiotica
fungi
Modalità d’azione
DNA synthesis | interferes
enzyme | inhibits
Temperatura di conservazione
−20°C
Stringa SMILE
Nc1ncnc2n(cnc12)[C@@H]3O[C@H](CO)C[C@H]3O
InChI
1S/C10H13N5O3/c11-8-7-9(13-3-12-8)15(4-14-7)10-6(17)1-5(2-16)18-10/h3-6,10,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,10+/m0/s1
OFEZSBMBBKLLBJ-BAJZRUMYSA-N
Informazioni sul gene
rat ... Adora1(29290)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.