Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
C2020
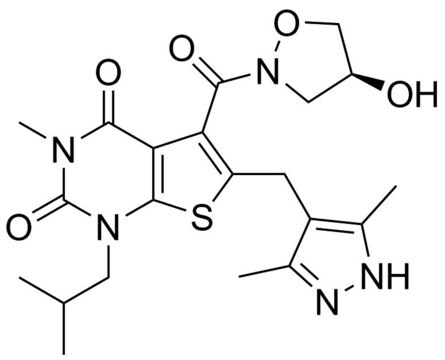
α-Cyano-4-hydroxycinnamic acid
≥98% (TLC), powder, monocarboxylic acid transport inhibitor
Sinonimo/i:
α-CCA, α-CHCA, α-Cyano, 4-HCCA, ACCA
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Nome del prodotto
α-Cyano-4-hydroxycinnamic acid, ≥98% (TLC), powder
Saggio
≥98% (TLC)
Stato
powder
Colore
yellow
Punto di fusione
245-250 °C (lit.)
Solubilità
H2O: slightly soluble
methanol: water: soluble
polar organic solvents: soluble
Temperatura di conservazione
2-8°C
Stringa SMILE
OC(=O)\C(=C\c1ccc(O)cc1)C#N
InChI
1S/C10H7NO3/c11-6-8(10(13)14)5-7-1-3-9(12)4-2-7/h1-5,12H,(H,13,14)/b8-5+
AFVLVVWMAFSXCK-VMPITWQZSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Skin Sens. 1B
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Contenuto correlato
ZipTip® micro-SPE pipette tips are used as a single-step desalting, concentration, and purification tool for complex samples before mass spec analyses.
-
What is the Department of Transportation shipping information for this product?
1 risposta-
Utile?
-
-
What can be used for solublization of α-Cyano-4-hydroxycinnamic acid?
1 risposta-
α-Cyano-4-hydroxycinnamic acid (α-CCA, α-CHCA, α-Cyano, 4-HCCA, ACCA) is soluble in methanol (up to 50 mg/ml). It is also soluble at 10 mg/ml using 50% acetonitrile in 0.05% TFA for MALDI-MS. The acetonitrile concentration can be adjusted for individual preferences.For biological applications, it has been solubilized at 100 mM in DMSO or 50 mM in ethanol.
Utile?
-
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.