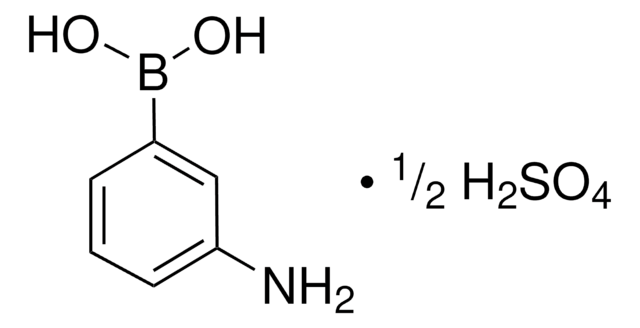
A8312
m-Aminophenylboronic acid–Agarose
aqueous suspension
Sinonimo/i:
(3-aminophenyl)boronic acid-Agarose, 3-Aminophenylboronic acid–Agarose, m-APBA-Agarose, m-Aminophenylboronic acid resin
About This Item
Prodotti consigliati
Forma fisica
aqueous suspension
Grado di funzionalizzazione
40-80 μmol per mL
Matrice
6% beaded agarose
Attivazione matrice
epichlorohydrin
Gruppi immobilizzati alla matrice
through amino to carboxyls of EDTA
Braccio spaziatore
9 atoms
Capacità
≥8 mg/mL binding capacity (peroxidase Type VI)
Temperatura di conservazione
2-8°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- as a component of the column for the purification of Escherichia coli TOP10F cells
- with cell lysates of various cells for detection of PARylated (Poly(ADP-ribos)ylation) proteins.
- in the affinity purification of horseradish peroxidase (HRPO)
- in the glycated human serum albumin (gHSA) purification
Stato fisico
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.