Y0001024
Ascorbic acid impurity C
European Pharmacopoeia (EP) Reference Standard
Sinonimo/i:
D-xylo-Hex-2-ulosonic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
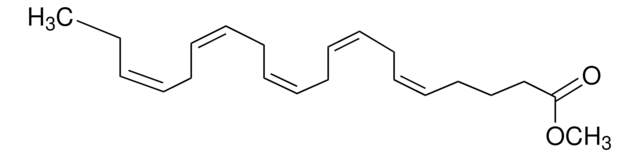
Formula empirica (notazione di Hill):
C6H10O7
Numero CAS:
Peso molecolare:
194.14
Codice UNSPSC:
41116107
NACRES:
NA.24
Prodotti consigliati
Grado
pharmaceutical primary standard
Famiglia di API
ascorbic acid
Produttore/marchio commerciale
EDQM
applicazioni
pharmaceutical (small molecule)
Formato
neat
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, has been developed and issued under the Authority of the Issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
Applicazioni
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia. Their suitability for any other use is not guaranteed and is the sole responsibility of the user. This standard is not intended for human or animal use.
Established for the preparation of reference solutions (a) in the testing of related substances in ascorbic acid and sodium ascorbate using liquid chromatography (General text 2.2.29), according to the monographs 0253 and 1791 of European Pharmacopoeia.
Established for the preparation of reference solutions (a) in the testing of related substances in ascorbic acid and sodium ascorbate using liquid chromatography (General text 2.2.29), according to the monographs 0253 and 1791 of European Pharmacopoeia.
Confezionamento
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Altre note
Sales restrictions may apply.
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Sodium Ascorbate
European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare
European pharmacopoeia, 6953-6954 (2022)
Ascorbic Acid
European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare
European pharmacopoeia, 6885-6887 (2022)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.