SMB01385
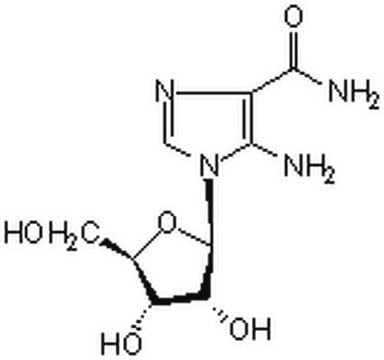
SAICAR
≥95% (HPLC)
Sinonimo/i:
N-[[5-amino-1-(5-O-phosphono-β-D-ribofuranosyl)-1H-imidazol-4-yl]carbonyl]-L-aspartic acid, Succino-AICAR, Succinyl-AICAR
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Saggio
≥95% (HPLC)
Forma fisica
solid
applicazioni
metabolomics
Condizioni di spedizione
2-8°C
Temperatura di conservazione
2-8°C
Stringa SMILE
NC1=C(N=CN1[C@H]2[C@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O2)C(N[C@@H](CC(O)=O)C(O)=O)=O
Descrizione generale
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.