N9890
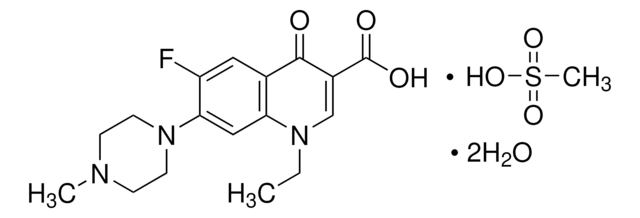
Norfloxacin
analytical standard, ≥98% (TLC)
Sinonimo/i:
1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid, 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-piperazino-3-quinolinecarboxylic acid
About This Item
Prodotti consigliati
Grado
analytical standard
Livello qualitativo
agenzia
EPA 1694
Saggio
≥98% (TLC)
tecniche
HPLC: suitable
gas chromatography (GC): suitable
applicazioni
clinical testing
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
CCN1C=C(C(O)=O)C(=O)c2cc(F)c(cc12)N3CCNCC3
InChI
1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23)
OGJPXUAPXNRGGI-UHFFFAOYSA-N
Informazioni sul gene
human ... CYP1A2(1544)
rat ... Gabra1(29705)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Mode of action: inhibits bacterial DNA replication
Antimicrobial spectrum: Gram-negative bacteria; less effective against Gram-positive bacteria
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Quinolones are a key group of antibiotics that interfere with DNA synthesis by inhibiting topoisomerase, most frequently topoisomerase II (DNA gyrase), an enzyme involved in DNA replication.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.