92330
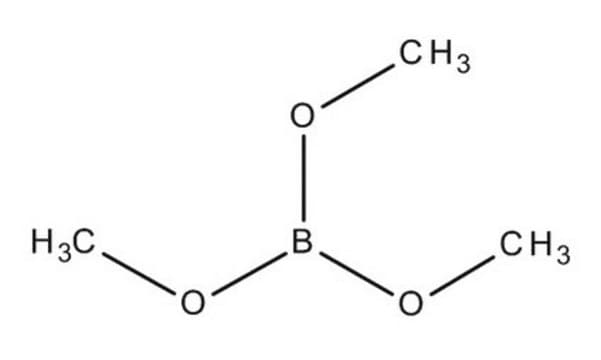
Trimethyl borate
purum, ≥99.0% (GC)
Sinonimo/i:
Boric acid trimethyl ester, Methyl borate
About This Item
Prodotti consigliati
Densità del vapore
3.59 (vs air)
Livello qualitativo
Grado
purum
Saggio
≥99.0% (GC)
Forma fisica
liquid
Indice di rifrazione
n20/D 1.346 (lit.)
n20/D 1.358
P. eboll.
68-69 °C (lit.)
Punto di fusione
−34 °C (lit.)
Densità
0.932 g/mL at 20 °C (lit.)
Stringa SMILE
COB(OC)OC
InChI
1S/C3H9BO3/c1-5-4(6-2)7-3/h1-3H3
WRECIMRULFAWHA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- For the synthesis of luminogens having triphenylamine core and tetraphenylethene peripheral moieties.
- For the synthesis of ammonia borane and trialkylamine boranes.
- In the reduction of carboxylic acids in the presence of borane-methyl sulfide.
- As a reagent to crosslink phopshate complexes.
- As a source of boron to synthesize boron nitride nanotubes by chemical vapor deposition (CVD) method.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Repr. 1B - STOT SE 1
Organi bersaglio
Eyes
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
12.2 °F - (own results)
Punto d’infiammabilità (°C)
-11 °C - (own results)
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.