W237809
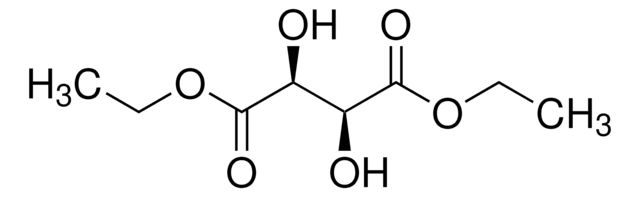
Diethyl L-tartrate
≥99%, FG
Sinonimo/i:
(+)-Diethyl L-tartrate, L-(+)-Tartaric acid diethyl ester
Scegli un formato
47,40 €
Scegli un formato
About This Item
47,40 €
Prodotti consigliati
Origine biologica
synthetic
Grado
FG
Fragrance grade
Kosher
agenzia
follows IFRA guidelines
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Saggio
≥99%
Attività ottica
[α]20/D +8.5°, neat
Indice di rifrazione
n20/D 1.446 (lit.)
P. ebollizione
280 °C (lit.)
Densità
1.204 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Organolettico
fruity; wine-like
Stringa SMILE
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
YSAVZVORKRDODB-PHDIDXHHSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
199.4 °F - closed cup
Punto d’infiammabilità (°C)
93 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.