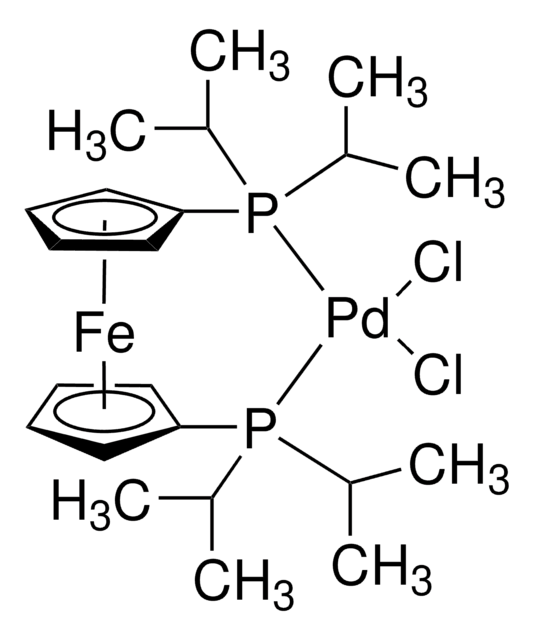
678740
(AMPHOS)2PdCl2
Sinonimo/i:
Bis[4-[Bis(tert-butyl)phosphino]-N, N-Dimethylbenzenamide]dichloropalladium
About This Item
Prodotti consigliati
Stato
solid
Livello qualitativo
Impiego in reazioni chimiche
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
Gruppo funzionale
phosphine
Stringa SMILE
Cl[Pd]Cl.CN(c1ccc(P(C(C)(C)C)C(C)(C)C)cc1)C.CN(c2ccc(P(C(C)(C)C)C(C)(C)C)cc2)C
InChI
1S/2C16H28NP.2ClH.Pd/c2*1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;;;/h2*9-12H,1-8H3;2*1H;/q;;;;+2/p-2
DWOZNANUEDYIOF-UHFFFAOYSA-L
Descrizione generale
For small scale and high throughput uses, product is also available as ChemBeads (927791)
Applicazioni
- In the enantioselective construction of indole-fused bicyclo[3.2.1]-octanes via an aminopalladition-triggered Heck-type reaction.
- In the synthesis of phenanthridine derivatives from ortho bromo N-tosylhydrazones and 2-aminophenylboronic ester via Suzuki cross-coupling reaction followed by intramolecular condensation reaction.
- In the synthesis of indenones by Pd-catalyzed annulation of an ortho-halobenzyl alcohol with internal alkynes.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Ligand used to prepare a palladium dichloride catalyst on treatment with PdCl2(COD). The catalyst effectively cross-couples aryl boronic acids with heteroaryl chlorides.
A variety of palladium-catalyzed cross-coupling reactions can be run under room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
Protocolli
TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)








![[1,1′-Bis(di-cyclohexylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/136/854/a3142b2e-900c-47e5-8100-e48add9f4db6/640/a3142b2e-900c-47e5-8100-e48add9f4db6.png)