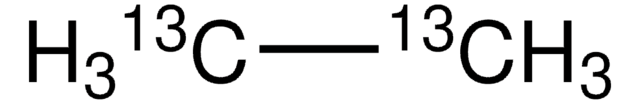539775
Ethane
99.99%
Sinonimo/i:
Ethane gas
About This Item
Prodotti consigliati
Densità del vapore
1.05 (vs air)
Tensione di vapore
37.95 atm ( 21.1 °C)
Saggio
99.99%
Forma fisica
gas
Temp. autoaccensione
881 °F
Limite di esplosione
13 %
P. eboll.
−88 °C (lit.)
Punto di fusione
−172 °C (lit.)
Densità
0.362 g/mL at 20 °C (lit.)
Stringa SMILE
CC
InChI
1S/C2H6/c1-2/h1-2H3
OTMSDBZUPAUEDD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- High-pressure oxidation of ethane: The paper discusses the oxidation properties of ethane under high pressure, providing insights crucial for developing combustion models and understanding ethane′s behavior in various industrial processes (H Hashemi, JG Jacobsen, CT Rasmussen, 2017).
- Progress and prospects in catalytic ethane aromatization: This review highlights the advancements in converting ethane to more valuable aromatic hydrocarbons, showcasing the potential of ethane as a petrochemical feedstock (Y Xiang, H Wang, J Cheng, J Matsubu, 2018).
Confezionamento
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Altre note
Note legali
Comunemente ordinati con questo prodotto
Portagomma
Raccomandato
Regolatore
Valvola di controllo
Valvola di spurgo
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Flam. Gas 1A - Press. Gas Liquefied gas
Codice della classe di stoccaggio
2A - Gases
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
-211.0 °F - closed cup
Punto d’infiammabilità (°C)
-135 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Separation of Methane; Acetylene; Carbon monoxide; Water; Nitrogen; Carbon dioxide; Ethane; Ethylene
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.