483451
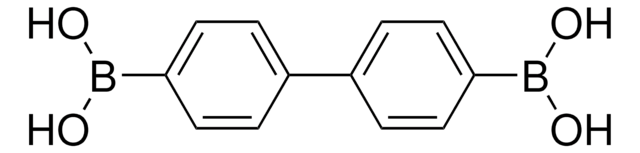
4-Biphenylboronic acid
≥95.0%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5C6H4B(OH)2
Numero CAS:
Peso molecolare:
198.03
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥95.0%
Punto di fusione
232-245 °C (lit.)
Stringa SMILE
OB(O)c1ccc(cc1)-c2ccccc2
InChI
1S/C12H11BO2/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,14-15H
XPEIJWZLPWNNOK-UHFFFAOYSA-N
Categorie correlate
Altre note
Contains varying amounts of anhydride
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Kazuhiko Tsukagoshi et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 23(2), 227-230 (2007-02-14)
In our previous study, we proposed molecular recognition of mono- and disaccharides making use of the interaction between their diol groups and p-iodophenylboronic acid in capillary electrophoresis with a chemiluminescence detection system. Here, to extend our knowledge of molecular recognition
L J Kricka et al.
Journal of bioluminescence and chemiluminescence, 11(3), 137-147 (1996-05-01)
The enhancers 1,1'-biphenyl-4-yl boronic acid and 4-iodophenol act synergistically in the horseradish peroxidase-catalysed oxidation of luminol. This concentration-dependent effect reduces background, increases signal and hence improves signal/background for detection of peroxidase. The same type of synergistic effect was found when
Anna Minkkilä et al.
Journal of medicinal chemistry, 51(22), 7057-7060 (2008-11-06)
A series of commercial phenyl-, heteroaryl-, alkyl-, and alkenylboronic acids were evaluated for their FAAH and MGL inhibitory activities. The compounds were generally selective for FAAH, with IC50 in the nanomolar or low-micromolar range. Eight of these compounds inhibited MGL
Steven R Inglis et al.
Journal of medicinal chemistry, 52(19), 6097-6106 (2009-09-08)
Penicillin binding proteins (PBPs) catalyze steps in the biosynthesis of bacterial cell walls and are the targets for the beta-lactam antibiotics. Non-beta-lactam based antibiotics that target PBPs are of interest because bacteria have evolved resistance to the beta-lactam antibiotics. Boronic
Gintare Krucaite et al.
Molecules (Basel, Switzerland), 26(7) (2021-04-04)
A group of polyethers containing electroactive pendent 4,7-diarylfluorene chromophores have been prepared by the multi-step synthetic route. Full characterization of their structures has been presented. The polymeric materials represent derivatives of high thermal stability with initial thermal degradation temperatures in
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.