465194
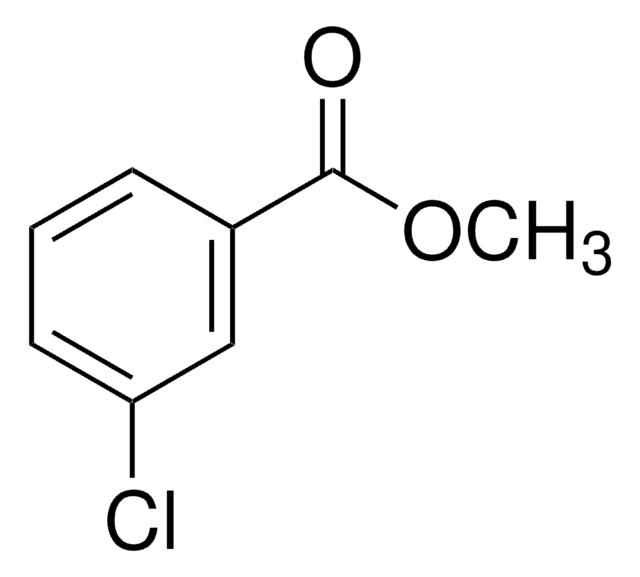
Methyl 2-chlorobenzoate
≥98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
ClC6H4CO2CH3
Numero CAS:
Peso molecolare:
170.59
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥98%
Stato
liquid
Indice di rifrazione
n20/D 1.536 (lit.)
P. ebollizione
86-88 °C/2.5 mmHg (lit.)
Densità
1.191 g/mL at 25 °C (lit.)
Stringa SMILE
COC(=O)c1ccccc1Cl
InChI
1S/C8H7ClO2/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5H,1H3
JAVRNIFMYIJXIE-UHFFFAOYSA-N
Descrizione generale
Methyl 2-chlorobenzoate, a methyl 2-halobenzoate, is an ester. It can be synthesized from 2-chlorobenzoyl chloride. Its reduction with NaBH4 in diglyme at 162°C affords 2-chlorobenzyl alcohol.
Applicazioni
Methyl 2-chlorobenzoate may be used in the synthesis of various quinazolinone derivatives. It was used as starting reagent in the synthesis of 2-chlorobenzohydrazide.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
228.2 °F - closed cup
Punto d’infiammabilità (°C)
109.00 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Navin B Patel et al.
Scientia pharmaceutica, 78(2), 171-193 (2010-12-24)
In attempt to find new pharmacologically active molecules, we report here the synthesis and in vitro antimicrobial activity of various 3-(1,3,4-oxadiazol-2-yl)-quinazolin-4(3H)-ones. The antimicrobial activity of title compounds were examined against two gram positive bacteria (S. aureus, S. pyogenes), two gram
Reductions of Carboxylic Acids and Esters with NaBH4 in Diglyme at 162?C.
Zhu H-J and Pittman Jr CU.
Synthetic Communications, 33(10), 1733-1750 (2003)
Cheng Huang et al.
Chemical communications (Cambridge, England), (47)(47), 6333-6335 (2008-12-03)
We have developed a general and highly efficient copper-catalyzed method for synthesis of quinazoline and quinazolinone derivatives, the target products were obtained in good to excellent yields via cascade reactions of amidine hydrochlorides with substituted 2-halobenzaldehydes, 2-halophenylketones, or methyl 2-halobenzoates
Nicholas R Larson et al.
Journal of medical entomology, 57(1), 187-191 (2019-09-10)
Common bed bug Cimex lectularius (L.) (Hemiptera: Cimicidae) infestations are on the rise and due to the development of pesticide resistance they are becoming more difficult to control, affordably. We evaluated a naturally occurring compound methyl benzoate (MB) and related
Yan Feng et al.
Scientific reports, 8(1), 7902-7902 (2018-05-23)
Benzyl methyl ester, also known as methyl benzoate (MB), is a volatile organic compound that exists naturally as a floral fragrance in many plants. Our behavioral bioassays show that MB and some of its naturally occurring and synthetic analogs kill
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.